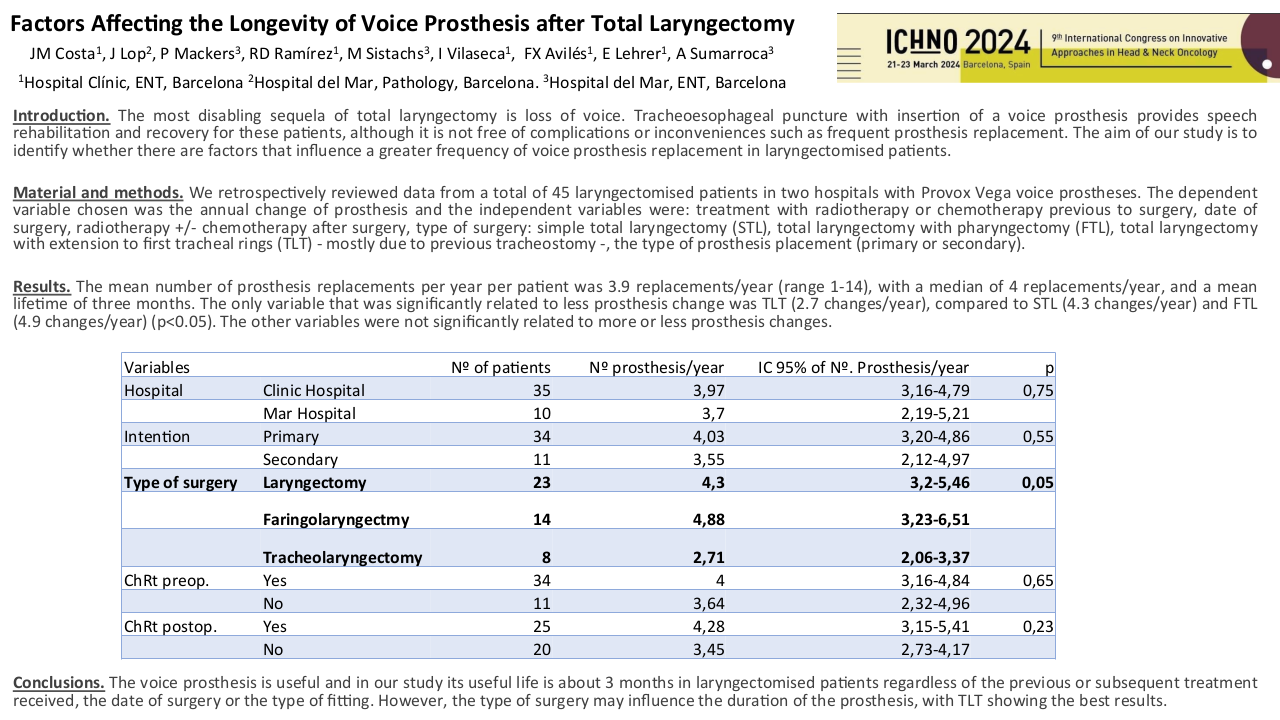
MuciLight trial : phase 2 of LED photobiomodulation for treatment of mucositis in oral cavity or oropharyngeal cancer.
Purpose/Objective
monocentric prospective phase 2 trial to evaluate the photobiomodulation with LED (PLED) lamp for curative treatment of mucositis in a high risk population without nutritionnal support : oral cavity or orophayngeal cancer treated by radiotherapy (RT) or radiochemotherapy in a postoperative or exclusive situation. PLED is a easy and fast technic to realize photobiomodulation with nurse or paramedical staff, with the whole oral cavity treated at once, and the exposable part of the oropharynx.
Material/Methods
Inclusion criteria were as follow : oral cavity or oropharyngeal cancer treated by radiation therapy or radiochemotherapy with at least one criteria of risk of grade 3 mucositis (G3M) : concomittant chemotherapy, activ tabagism, denutrition or diabetes ; grade 1 ou 2 mucositis (G1M G2M) ; age above 18 years old. PLED has to begin during week 2 or 3 during RT, and PLED scheme constited of a daily session of 13 min of 630 nm with 75 J/cm3 of PLED with Medisol® lamp. Clinical evaluation (pain, nutrition, PS) were performed daily with a nurse, and every week with the radio-oncologist until the end of RT and one month after the end of RT. Primary objective was the non occurrence of G3M, scored by CTCAE v5 during the beginning of PLED and the end of RT. The non occurrence of G3M was defined as a success of the PLED. Secondary objectives were pain evaluation, type of pain killers prescribed, quality of life (QoL), safety of PLED, weight variation and indication of enteral nutrition. 50 % of G3M was considered as the null hypothesis, and, with a proportion of success anticipated at 75 %, 26 patients were needed.
Results
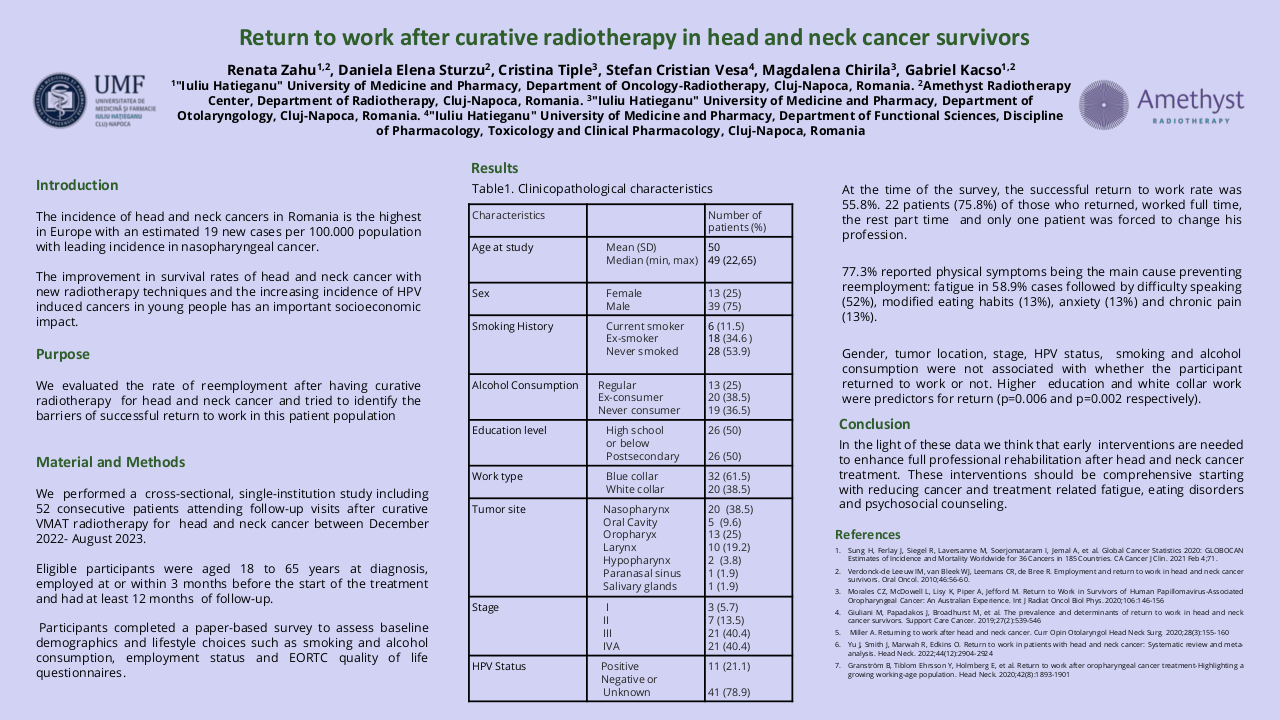
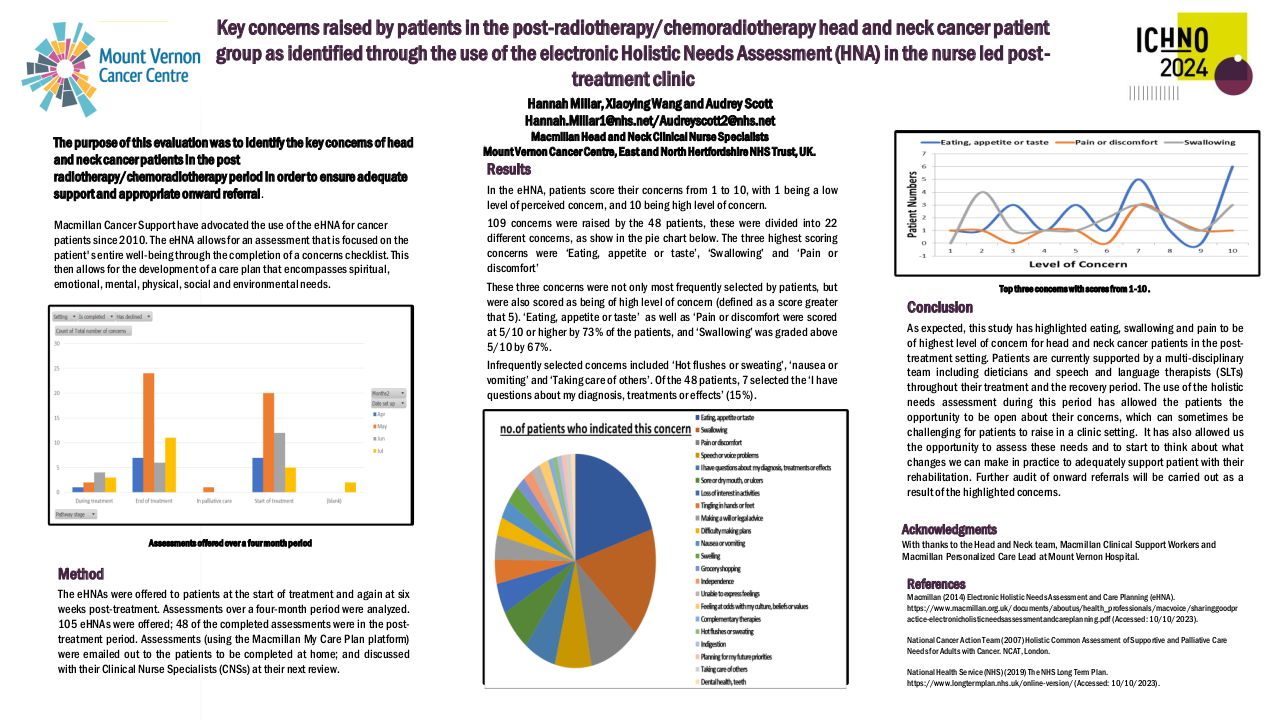
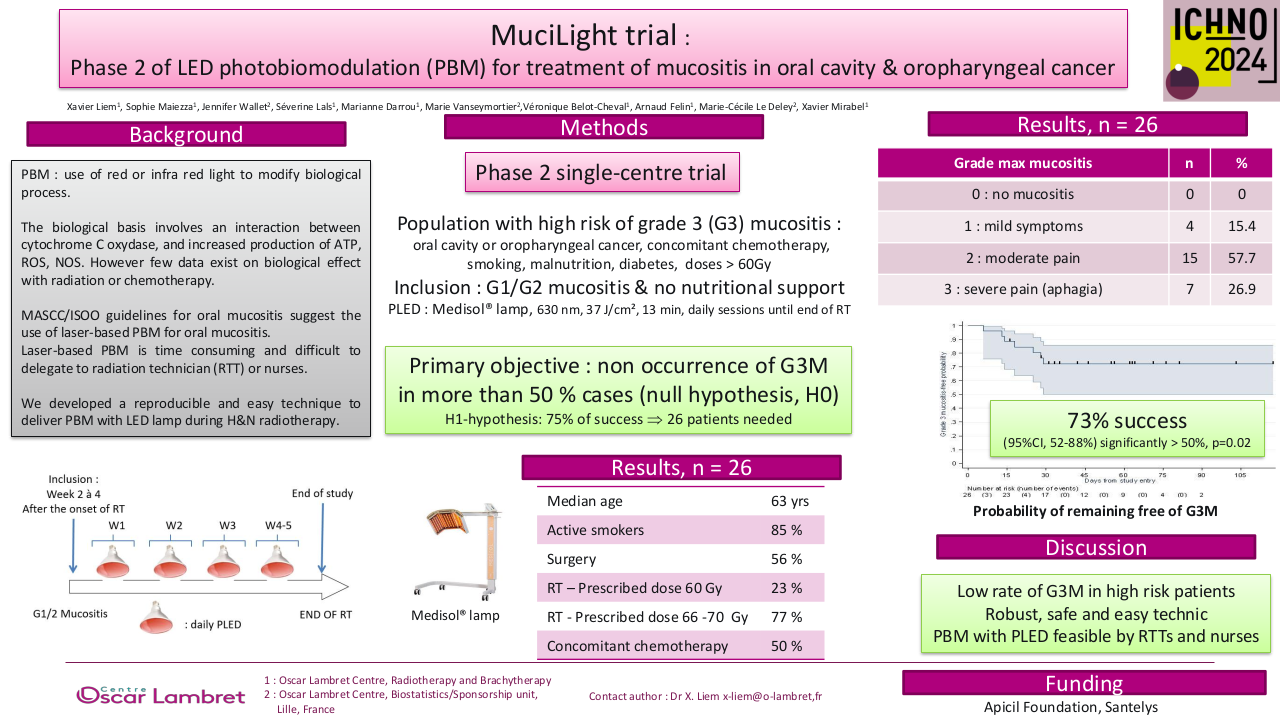
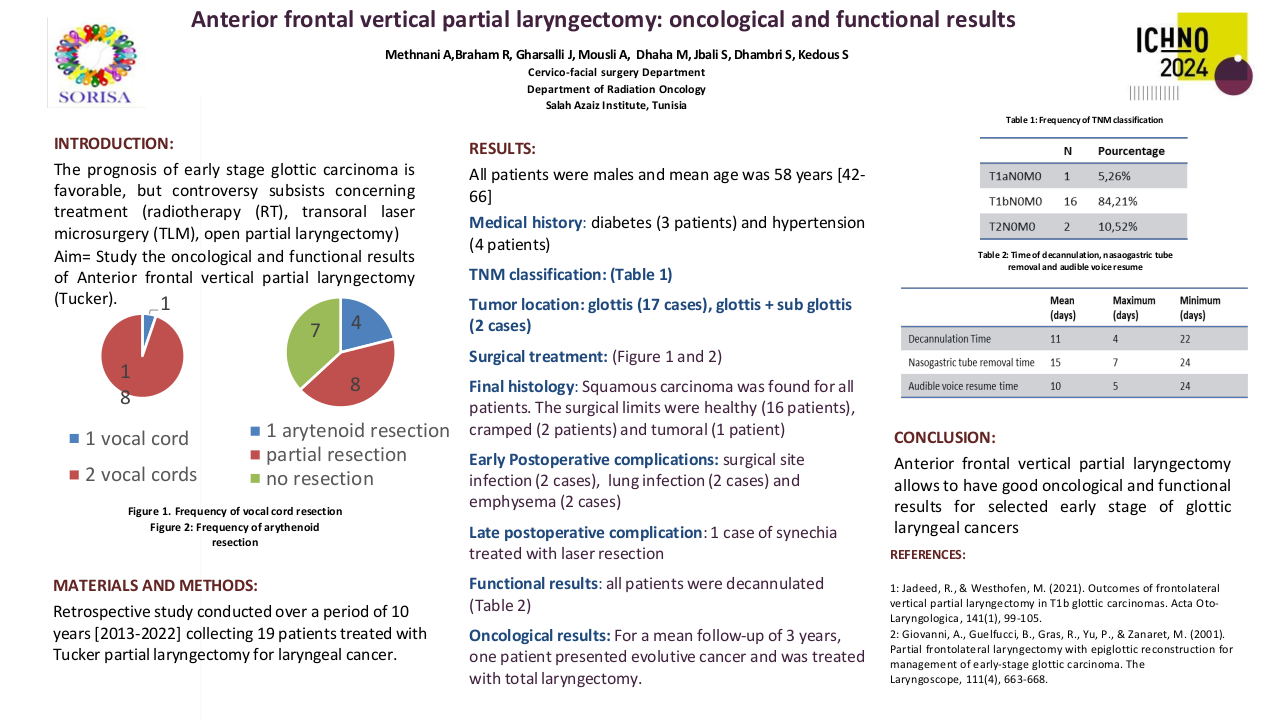
26 patients were included from mars 2021 to august 2022. Median age was 63, 85 % were active smokers and 15 % have already opiods prescription at the inclusion. 56 % received surgery before the radiation, 50 % of the patients had chemotherapy and all of the patients were prescribed more than 60 Gy. PLED was initated at 23 Gy in median, 85 % of patients had grade 1 mucositis (G1M) of the inclusion. 19 PLED session were realized (median) per patient, 2 patients stopped the PLED. 57 % had at least one temporary interruption of one session. At the end of the RT, 7 patients had G3M (27%), 11 G2M (42%), 6 G1M (23%) et 2 G0M (8%). Non occurend of G3M was 73 % (IC : 52 – 88 %, p = 0.019) at the end of RT and 72.2 % (IC 95 % 50.2 – 85.7% p <0.05). One patient had nasogastric tube one months after the end of RT. We observed a median weight variation of – 4 kg at the end of RT, which is below the threshold of denutrition definition. PS and Qol with QLQC30 and HN35 were statistically lower compared to baseline, in relation with the RT. 38 % of the patients had a new prescription of opoids. All patients experienced dry mouth, and 39 % had tingling sensation. No patient had G2 or more adverse effect of PLED.
Conclusion
With a 73 % of success (non occurrence of G3M) in a population of patients with high risk of severe oral mucositis without nutritional support, Mucilight trial reached his primary objective. Weight variation was below the 5 % variation theshold of denutrtion defined by the French health authorities. PLED is a safe and efficient technic of curative treatment of mucositis, and it’s easily feasible by nurse or paramedical staff.