The role of PD-L1 and TILsCD8+ in oral cavity and pharyngeal cancers treated with definitive radiation
Purpose/Objective
The incidence of head and neck cancer (HNC) which is the seventh most common cancer worldwide, continues to rise due to tobacco-derived carcinogens, excessive alcohol consumption, and common infection with oncogenic strains of human papillomavirus (HPV) [1,2]. The steady increase in the number of HPV-related oropharyngeal cancers makes the landscape of HNC more heterogeneous. Radiation therapy (RT) remains the mainstay of treatment as a sole modality, in combination with chemotherapy (chemoradiotherapy, CRT) or adjuvant after surgery. However, reliable predictive markers of response to conventional RT or CRT have not been found, despite established knowledge about the predictors of checkpoint inhibitors in metastatic disease. In view of the unsatisfactory results of clinical trials using concurrent RT/CRT with immunotherapy, increasing attention is being paid to the local effects of RT on the tumor microenvironment and systemic immune response. We hypothesized that the baseline expression of programmed death 1 ligand (PD-L1) and intratumoral infiltration with CD8+ lymphocytes (TILsCD8+) score in the non-metastatic oral cavity (OC) and oro-/hypopharynx (PX) cancers may correlate with RT response.
Material/Methods
It was a retrospective single-center analysis involving OC and PX patients who underwent radical RT (66Gy prescribed to the primary tumor, given in 30 fx/6 weeks) with or without concurrent chemotherapy (Cisplatin 100 mg/m2 i.v. every three weeks or 40 mg/m2 i.v weekly). We performed analysis of TILsCD8+ and PD-L1 expression status in the archival biospecimens of the treatment-naïve primary tumor. Tissue microarrays (TMA) were prepared using the Manual Tissue Arrayer MTA-1 (Beecher Instruments, Inc., USA). The TMA sections were first stained with hematoxylin and eosin to verify the invasive neoplastic content within each core. Next, consecutive sections were stained with IVD-grade antibodies, anti-PD-L1 (SP263) and anti-CD8 (SP57). PD-L1 expression was assessed in tumor cells, as TPS. For statistical analysis, the patients were divided into three groups: consistently negative (all cores with no positive cells), heterogeneous, and consistently PD-L1 high (const-high, all cores with >30% positive cells). Next, for each patient, the mean number of TILsCD8 per 1.76 mm2 of invasive tumor was calculated and recorded semi-quantitatively. Scores of <5, 6–50, 51–199, and ≥200 lymphocytes per core were rated as immunoscores (IMs) of 0, 1, 2, and 3, respectively. For statistical analyses, patients were divided into two groups: TILsCD8-negative (IM = 0) and TILsCD8-positive (IM ≥ 1).
Results
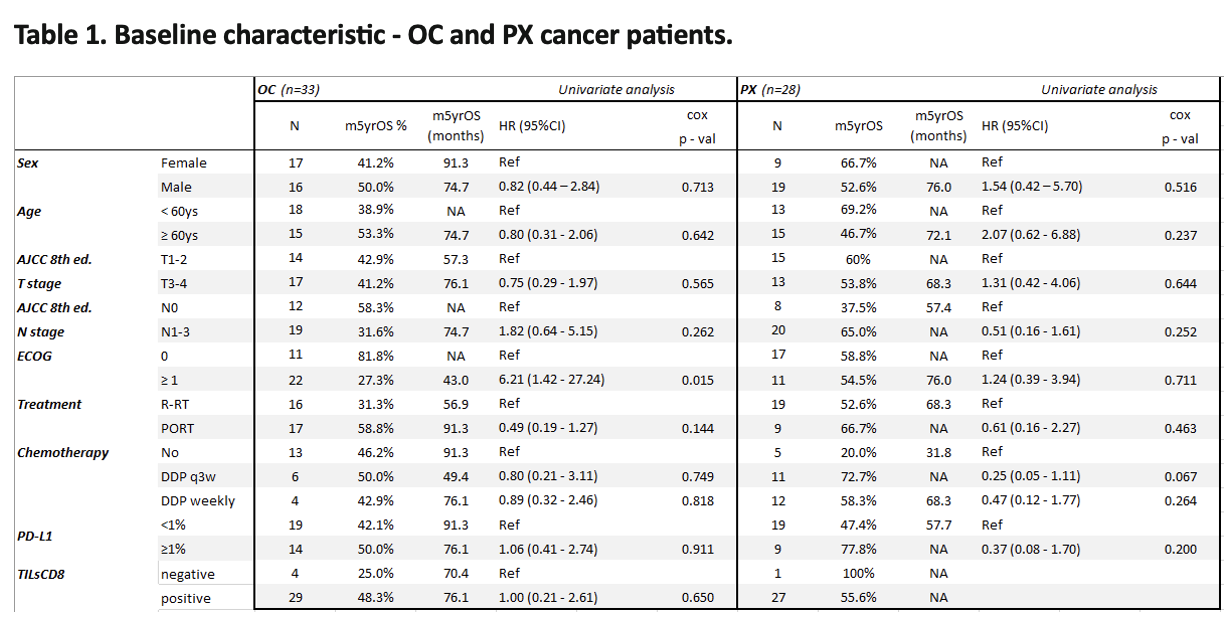
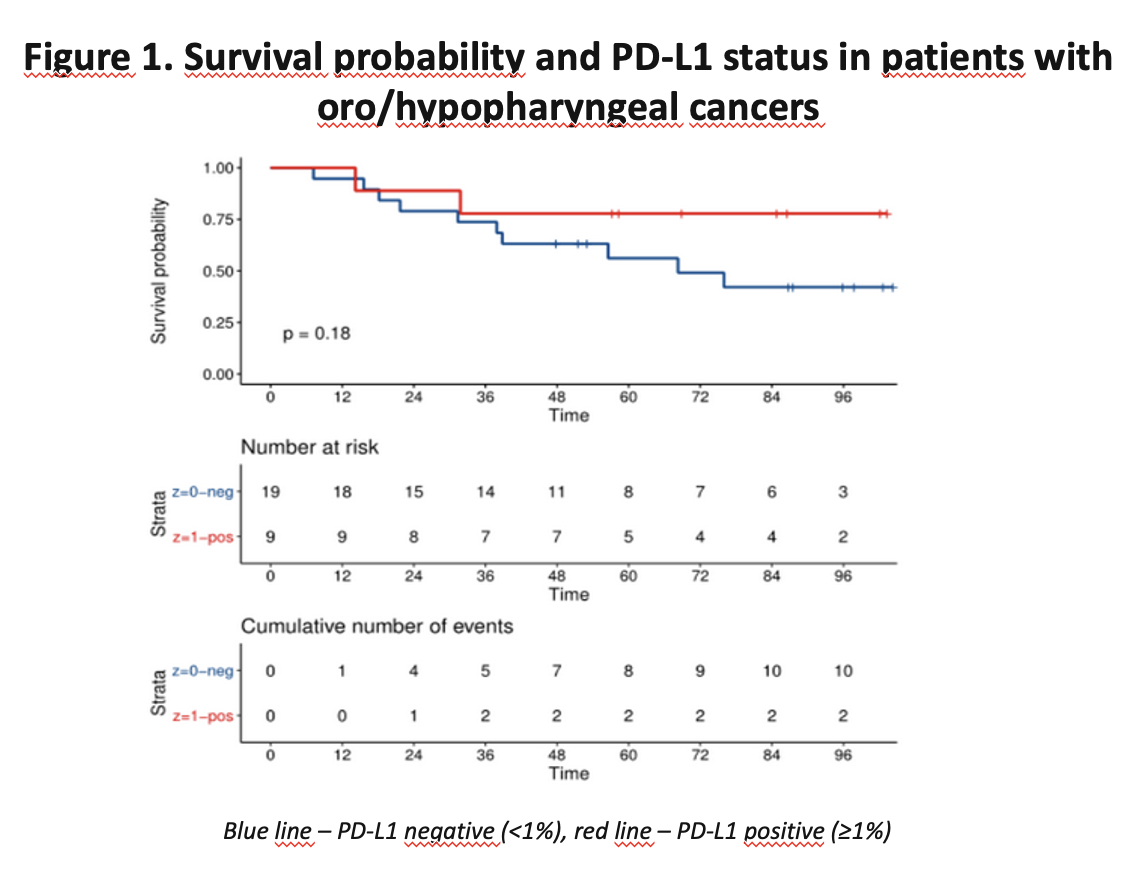
The study group was compromised of 61 patients (OC, n=33; PX, n=28). The cumulative 5-year overall survival (5YOS) rate was 57.58% and 64.29%, respectively. Patients were treated between 2012 and 2018, postoperatively (PORT; n=26) or qualified for definitive CRT (n=35). HPV-positive cancers accounted for 3% of OC and 54% of PX. In the OC and PX groups, more patients were PD-L1 negative, 57% and 67%, respectively (Table 1). The univariate analysis showed no influence of PD-L1 expression values on long-term survival. However, the survival curves of patients with PX and PD-L1 expression ≥1% vs. <1% visibly separate (p=0.18) (Figure 1). Most of the examined patients with OC showed a positive TILsCD8 score, with longer 5YOS compared to patients with score 0, however the difference did not reach statistical significance (OC - 48.3% vs 25%; p=0.65).
Conclusion
In our analysis, no association of PD-L1 expression and TILsCD8+ score, and patient outcome can be found. In univariate analysis we did not find any predictive factors for RT response. The main limitation of our study was very small group sizes.
1. Mody MD, Rocco JW, Yom SS, et al: Head and neck cancer. Lancet 398:2289-2299, 20212. https://gco.iacr.fr/overtime/en. Accessed October 10th, 2023