Transcriptomic and tumor microenvironment landscape of Epstein-Barr virus related Nasopharyngeal Carcinoma in endemic and non-endemic areas.
Purpose/Objective
Epstein-Barr virus (EBV)-related nasopharyngeal carcinoma (NPC) is an epithelial malignancy arising from the nasopharyngeal mucosal lining. A high incidence was recorded in EBV-endemic areas (EA) such as East and Southeast Asia [1] while in Europe, a non-endemic area (NEA), is low (1/105/year); however, the estimated survival rate is much lower than that recorded in Asian EA (5-year age-standardized relative survival = 54–57% vs. 74%) [2]. Risk factors of NPC include genetic, ethnic and environmental factors [3]. Differences in incidence and survival rates between EA and NEA NPCs could involve several factors, including EBV-related factors, genetic susceptibility of the population to EBV infections, and environmental factors such as diet and pollution [4-7]. Nevertheless, all proposed models of NPC pathogenesis are based on data derived from EA in Asia. Furthermore, clinical, pathogenic, and microenvironmental characteristics may play additional roles. EBV-related NPC in EA has already been characterized using genomic and transcriptomic data analysis [8-9]. However, gene expression analysis data [10] of NEA NPC is limited. Comparing gene expression data from EA and NEA diseases allows the recognition of similarities and differences in incidence and outcome among diseases arising in different geographical areas. We investigated the transcriptomic patterns of genes involved in EA NPC to interpret these differences and verifying them to an Italian cohort with available tumor tissue and clinical data. The immune and biological/functional characterization of EA and NEA NPC could improve the identification of new therapeutic strategies. Currently, the treatment for localized NPC includes radiotherapy, which is often combined with platinum-based chemotherapy, especially for locally advanced cancer. Neoadjuvant chemotherapy with cisplatin and gemcitabine was administered in the case of high-risk disease [11-12]. Immunotherapy with checkpoint inhibitors has shown clinical efficacy in recurrent/metastatic advanced NPC and is currently under evaluation to define its mechanism of action [13]. Our study aimed to dissect the gene expression (GE) and microenvironment of NPC, leading to the identification of the molecular subtypes of EA and NEA NPC. We also aimed to elucidate the biological and functional differences within EA NPC and between EA NPC and NEA NPC to eventually provide new insights into novel treatment strategies.
Material/Methods
Six GE datasets of NPC-EA transcriptomic repositories, including tumor and normal samples (GSE12452, GSE34573, GSE132112, GSE53819, GSE68799, GSE102349) and one validation dataset including both EA and NEA (https://doi.org/10.5281/zenodo.5347891) were retrieved. Four GE signatures associated to EBV related NPC prognosis (PMID: 24297049, 35262435, 32596151, 33096113), genes/pathways and gene sets (PMID: 35846746, 35394843, 35105963) were applied on EA and NEA NPC cohorts (Liu_NPC, Wood EBV EBNA1 Targets Down, Sengupta NPC_with LMP1 UP, REACTOME DNA Repair; Hallmarks). A bioinformatic meta-analysis approach was used to integrate the six EA datasets, and the classifier method was applied to the validation dataset in order to identify the subtype with worst prognosis. Moreover, RNA sequencing was performed on 50 Italian NEA NPC samples (study number: INT188/19; GSE208281). Biological and functional profiling of EA and NEA were performed using xCell, Gene set enrichment analyses, and treatment prediction methods (radiosensitivity index PMID: 16103067, pRRophetic R package, Immunophenoscore PMID: 28052254).
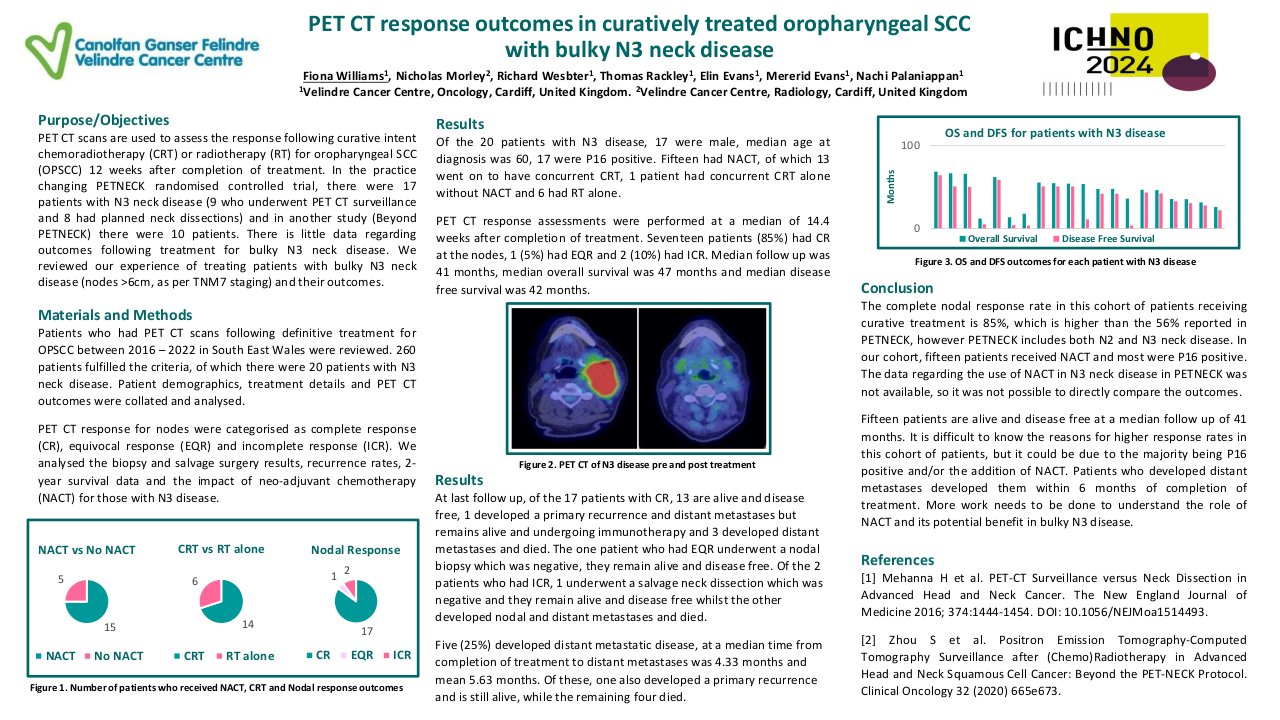
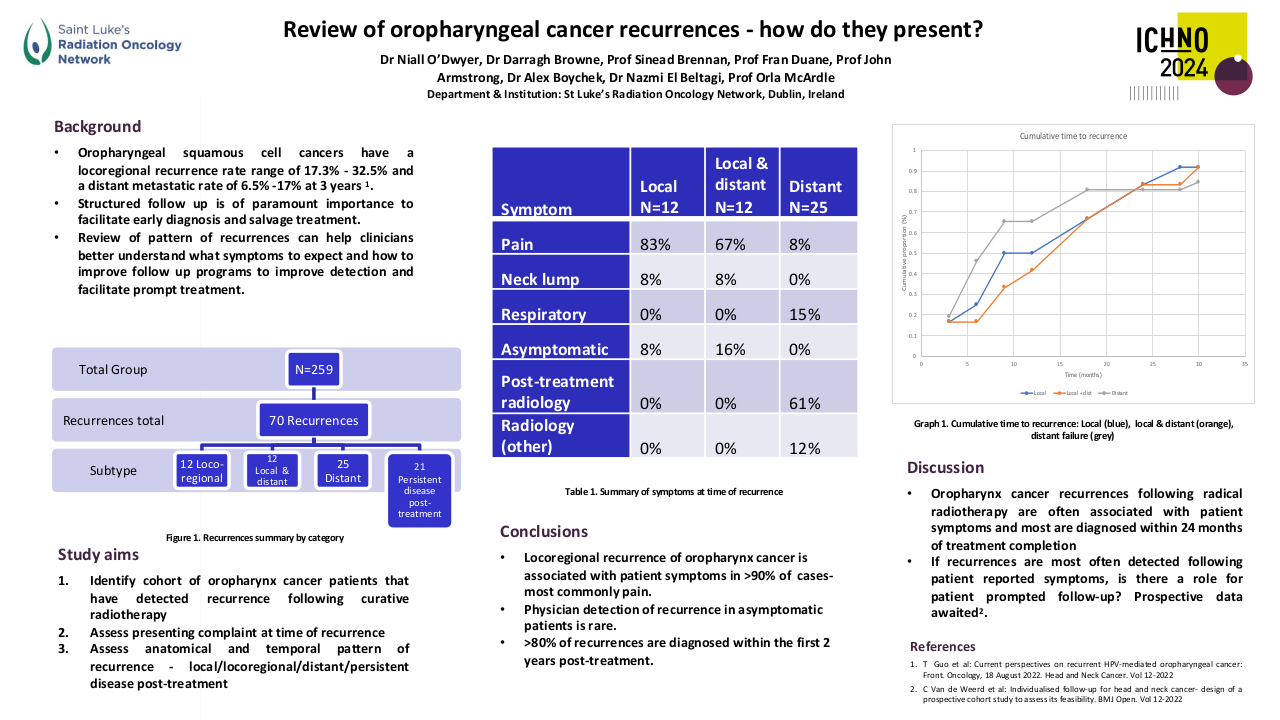
Results
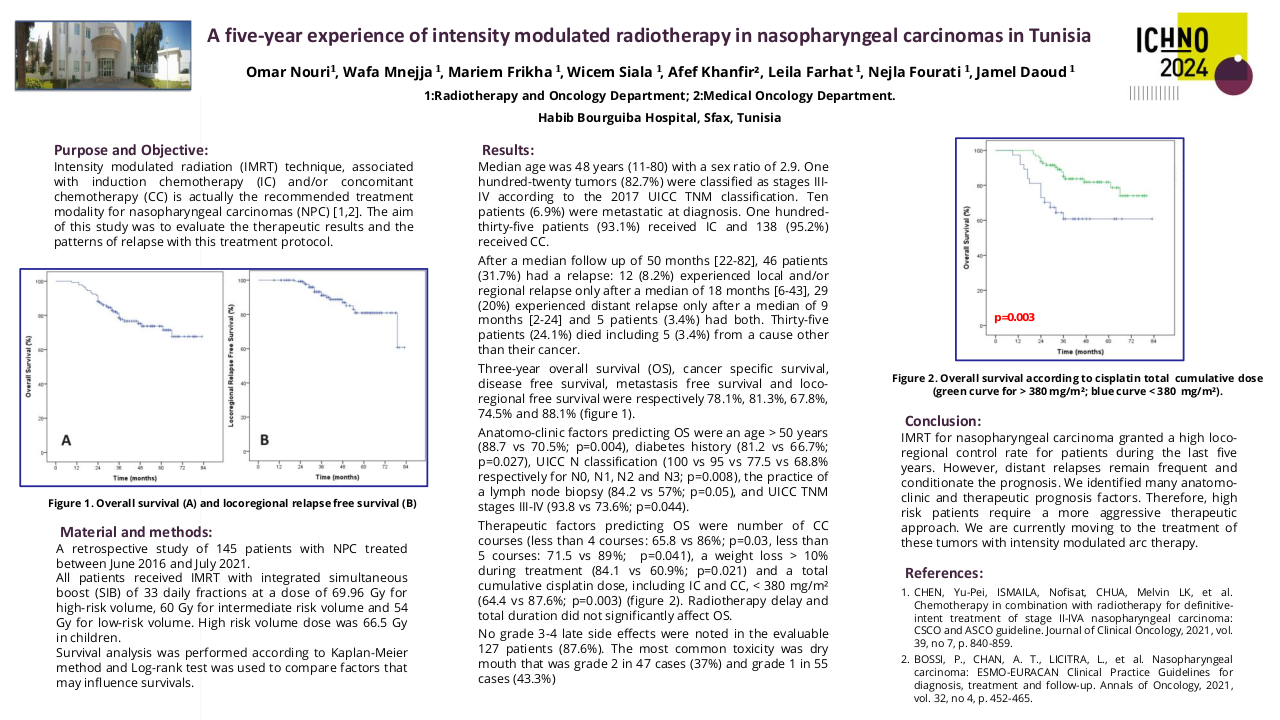
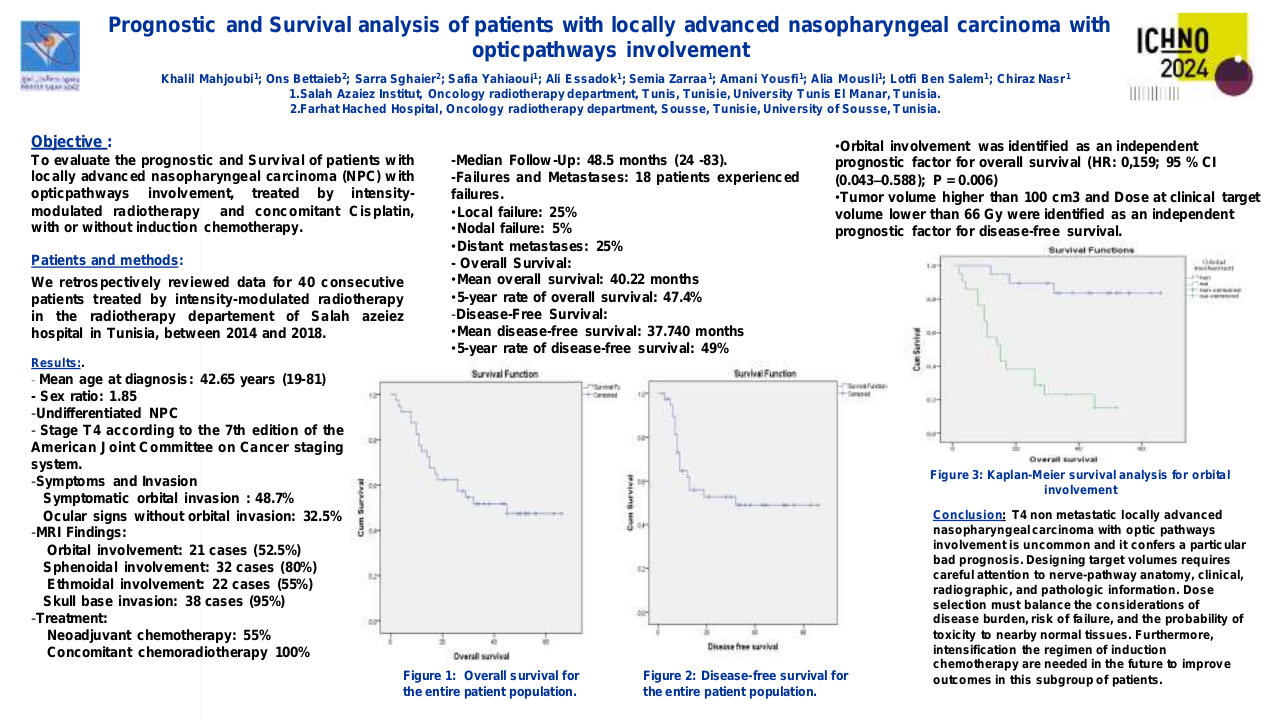
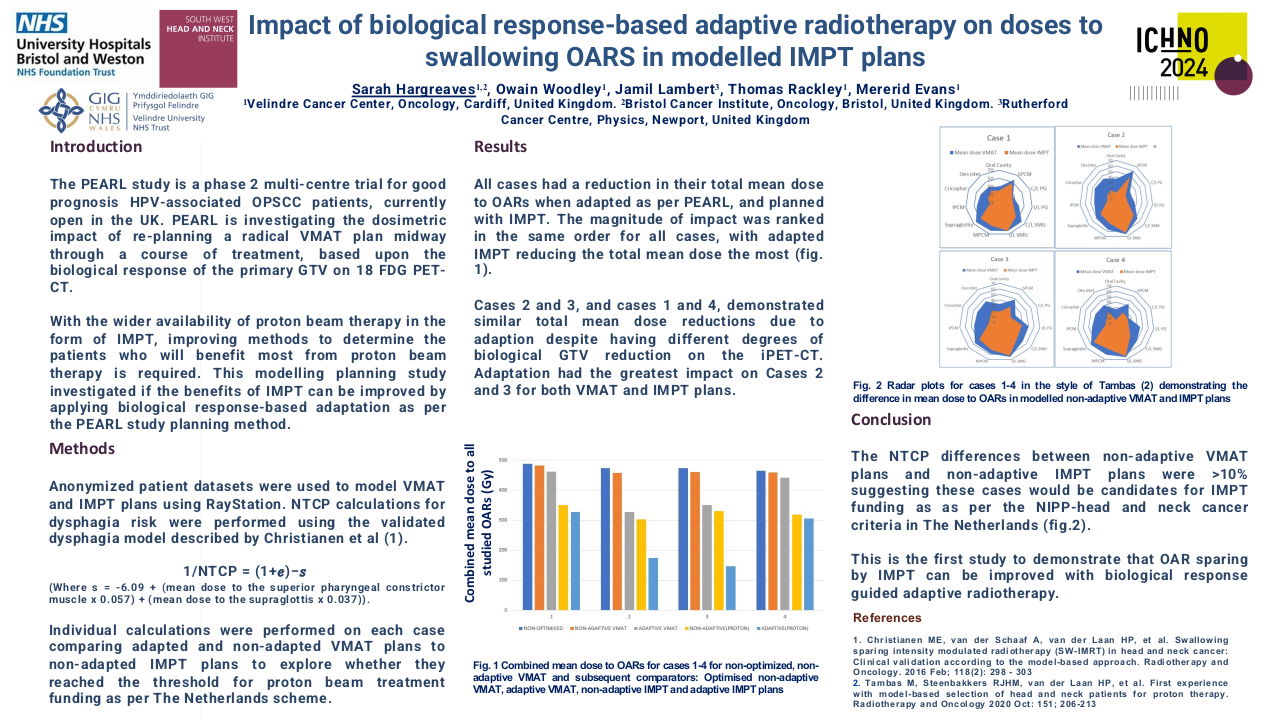
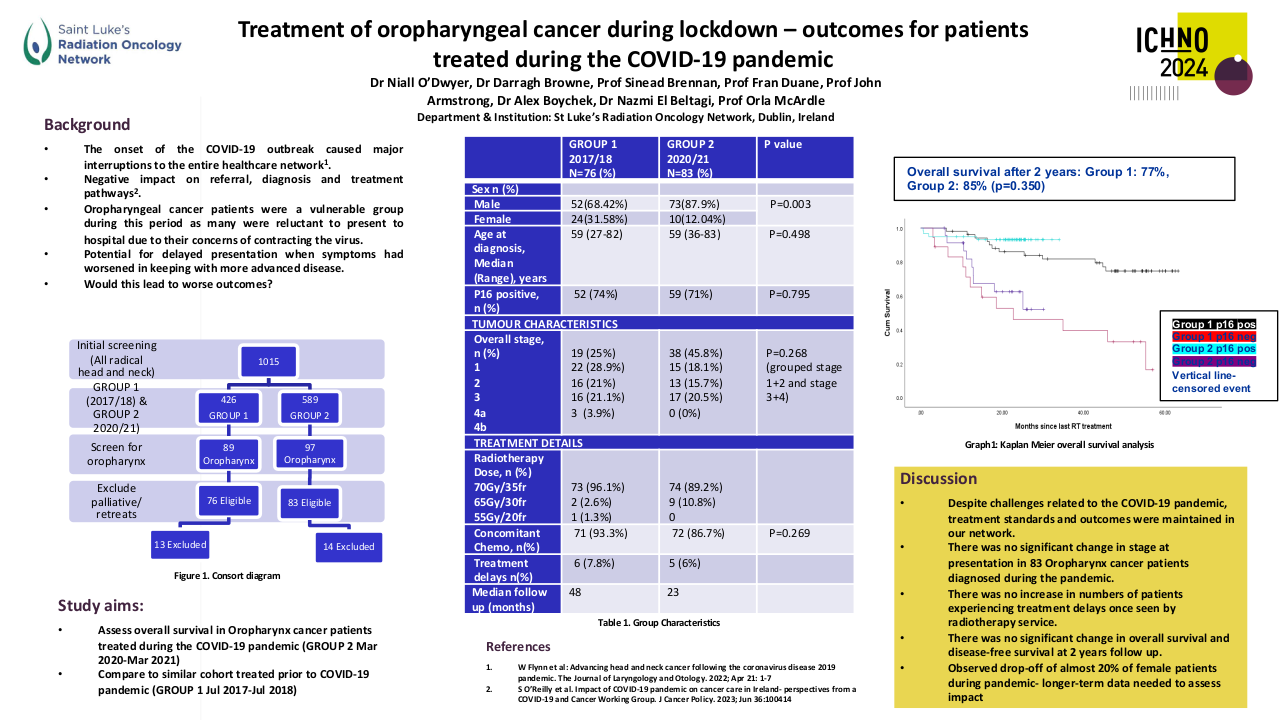
Through the meta-analysis of EA-NPC datasets, four clusters (Cl) were identified. Prognostic analyses revealed that Cl3 had the worst prognosis (P=0.0476), confirmed by three of the four prognostic signatures and in the validation dataset (P=0.0368). The biological and functional characterization of these clusters disclosed the relative GE subtypes: Cl1, Immune-active; Cl2, Defense-response; Cl3, Proliferation; Cl4, Perineural-interaction/EBV-exhaustion. According to the treatment prediction methods, the sensitivity of each cluster was radiotherapy and immunotherapy for immune-active, radiochemotherapy and immunotherapy for defense-response, chemotherapy for proliferation, and cisplatin treatment for perineural-interaction/EBV-exhaustion. Only three clusters ,excluding perineural-interaction/EBV-exhaustion, were expressed in our NEA cohort. Immune/biological characterization and treatment prediction analyses of NEA partially replicated the EA results.
Conclusion
Our study provides a relevant biological overview of EBV-related NPC in both EA and NEA. The immune microenvironment plays a critical role in NPC owing to the viral etiology of this malignancy. The presence of a perineural-interaction/EBV-exhaustion cluster in EA suggests an inactive EBV infection according to the viral related “hit and run theory”. Evaluation of miRNAs is ongoing along with miRNA/gene expression integration. Well characterized EA- and NEA-NPC retrospective and prospective cohorts are needed to validate the obtained results and can help designing future clinical studies.
1.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019 Jul 6;394(10192):64-80. doi: 10.1016/S0140-6736(19)30956-0. Epub 2019 Jun 6. PMID: 31178151.2. Gatta G, Capocacia R, Botta L, et al. Burden of centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol 2017; 18: 1022-393. Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell. 2004 May;5(5):423-8. doi: 10.1016/s1535-6108(04)00119-9. PMID: 15144950.4. Russo A, Crosignani P, Berrino F, et al. Incidence of cancer in migrants: data of the Lombardy tumor registry. Epidemiol Prev. 1994;18:125-32.5. Murata T, Sato Y, Kimura H. Modes of infection and oncogenesis by the Epstein-Barr virus. Rev Med Virol. 2014.6. Farrow DC, Vaughan TL, Berwick M, Lynch CF, Swanson GM, Lyon JL. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998 Dec 9;78(6):675-9. doi: 10.1002/(sici)1097-0215(19981209)78:6<675::aid-ijc2>3.0.co;2-j. PMID: 9833758. 7.Wang Y, Zhang Y, Ma S. Racial differences in nasopharyngeal carcinoma in the United States. Cancer Epidemiol. 2013 Dec;37(6):793-802. doi: 10.1016/j.canep.2013.08.008. Epub 2013 Sep 12. PMID: 24035238; PMCID: PMC3851929.8. Tsao SW, Tsang CM, Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. 2017 Oct 19;372(1732):20160270. doi: 10.1098/rstb.2016.0270. PMID: 28893937; PMCID: PMC5597737.9. Islam KA, Chow LK, Kam NW, Wang Y, Chiang CL, Choi HC, Xia YF, Lee AW, Ng WT, Dai W. Prognostic Biomarkers for Survival in Nasopharyngeal Carcinoma: A Systematic Review of the Literature. Cancers (Basel). 2022 Apr 24;14(9):2122. doi: 10.3390/cancers14092122. PMID: 35565251; PMCID: PMC9103785.10. Fountzilas G, Psyrri A, Giannoulatou E, Tikas I, Manousou K, Rontogianni D, Ciuleanu E, Ciuleanu T, Resiga L, Zaramboukas T, Papadopoulou K, Bobos M, Chrisafi S, Tsolaki E, Markou K, Giotakis E, Koutras A, Psoma E, Kalogera-Fountzila A, Skondra M, Bamia C, Pectasides D, Kotoula V. Prevalent somatic BRCA1 mutations shape clinically relevant genomic patterns of nasopharyngeal carcinoma in Southeast Europe. Int J Cancer. 2018 Jan 1;142(1):66-80. doi: 10.1002/ijc.31023. Epub 2017 Sep 30. PMID: 28857155.11. Bossi P, Chan AT, Licitra L, Trama A, Orlandi E, Hui EP, Halámková J, Mattheis S, Baujat B, Hardillo J, Smeele L, van Herpen C, Castro A, Machiels JP; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org; EURACAN. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2021 Apr;32(4):452-465. doi: 10.1016/j.annonc.2020.12.007. Epub 2020 Dec 25. PMID: 33358989.12. Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381(12):1124-1135.13. Xu JY, Wei XL, Wang YQ, Wang FH. Current status and advances of immunotherapy in nasopharyngeal carcinoma. Ther Adv Med Oncol. 2022 May 7;14:17588359221096214. doi: 10.1177/17588359221096214. PMID: 35547095; PMCID: PMC9083041