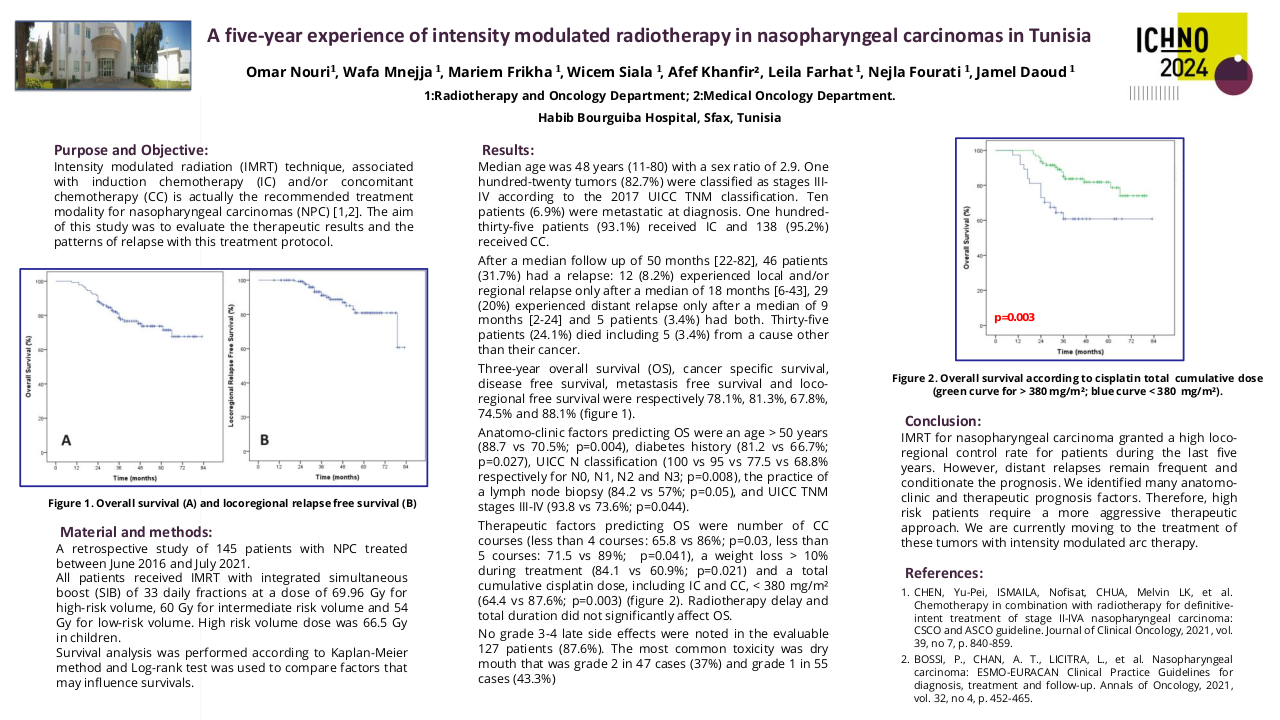
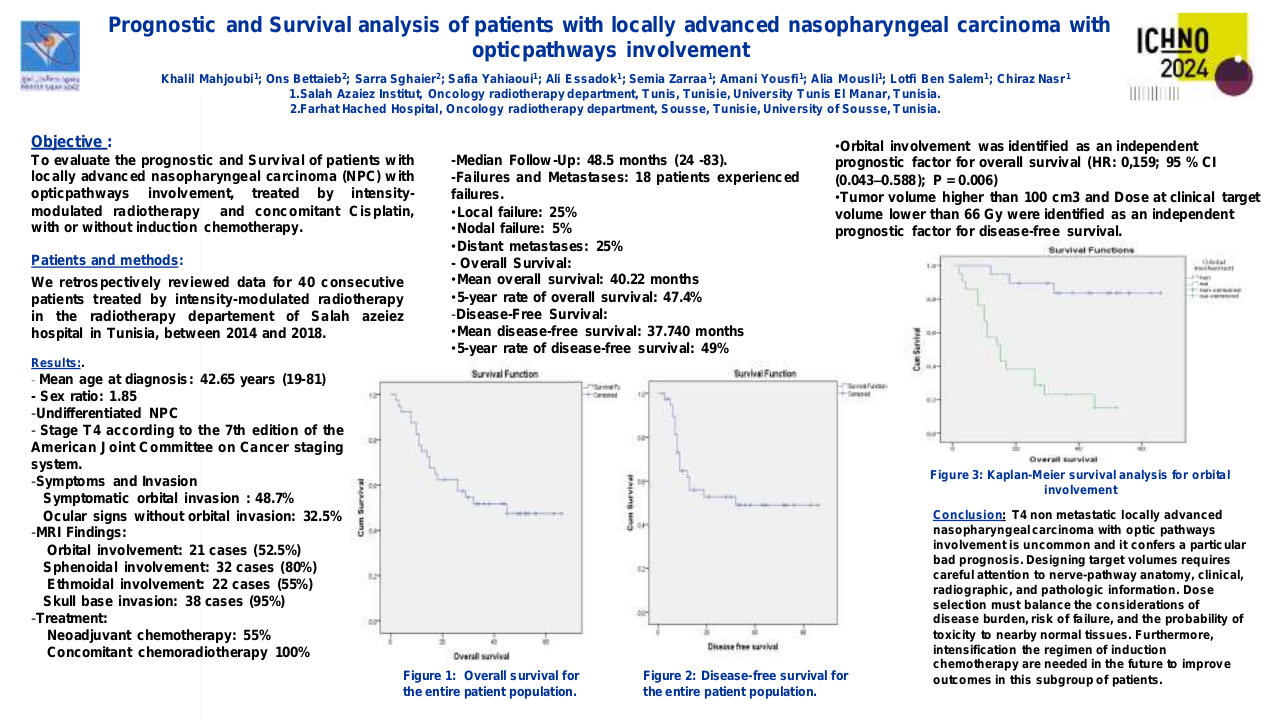
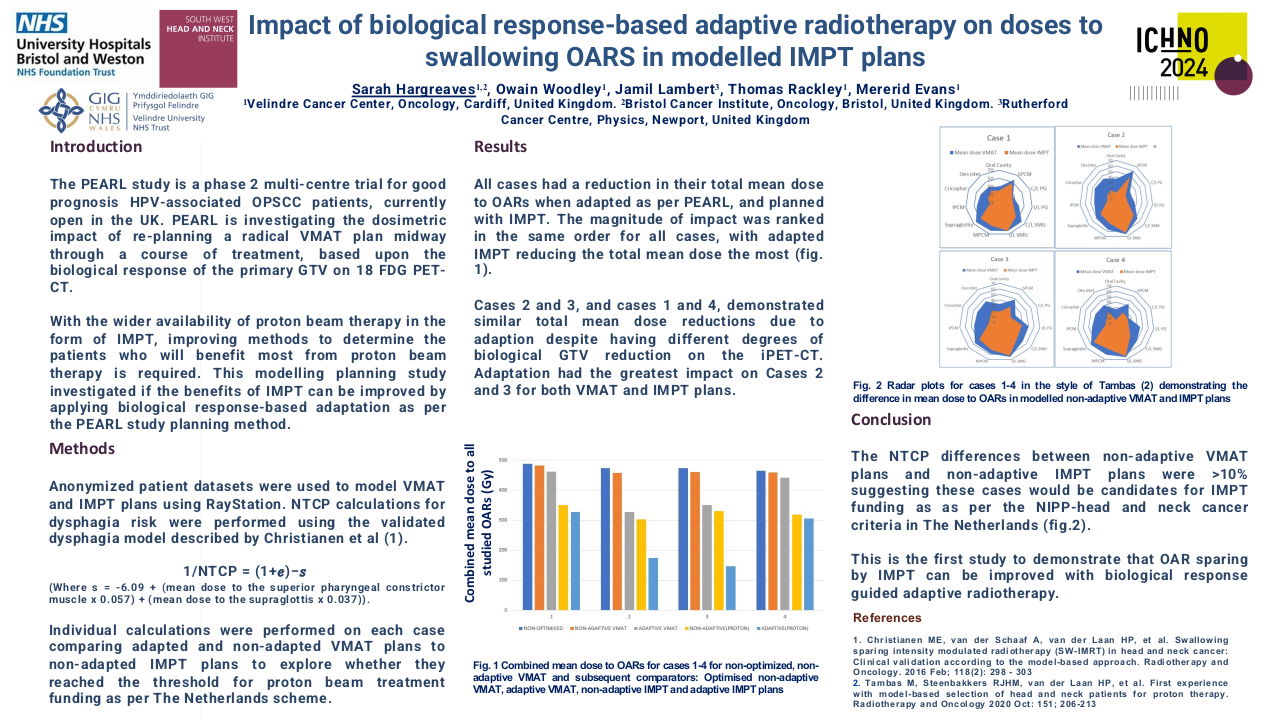
Impact of antibiotic use in the primary treatment of nasopharyngeal carcinoma
Purpose/Objective
Patients with nasopharyngeal carcinoma (NPC) benefit from antibiotics when there are infective complications during and around chemoradiotherapy, but there is some indication that antibiotics used peri treatment may affect patients’ outcomes. Here we sought to elucidate the exact impacts of antibiotics on NPC patients’ outcomes around primary treatment.
Material/Methods
This was a retrospective cohort study in a tertiary academic center in Hong Kong SAR. NPC patients treated for primary disease between 2010 and 2014 were evaluated for the impact of antibiotics prescribed around primary treatments on recurrence-free survival (RFS), disease-specific survival (DSS), and overall survival (OS), which was analyzed by multivariate Kaplan–Meier and Cox Proportional Hazard Regression analysis. The data were analyzed and performed with SPSS, V27.0 and GraphPad, V8.0
Results
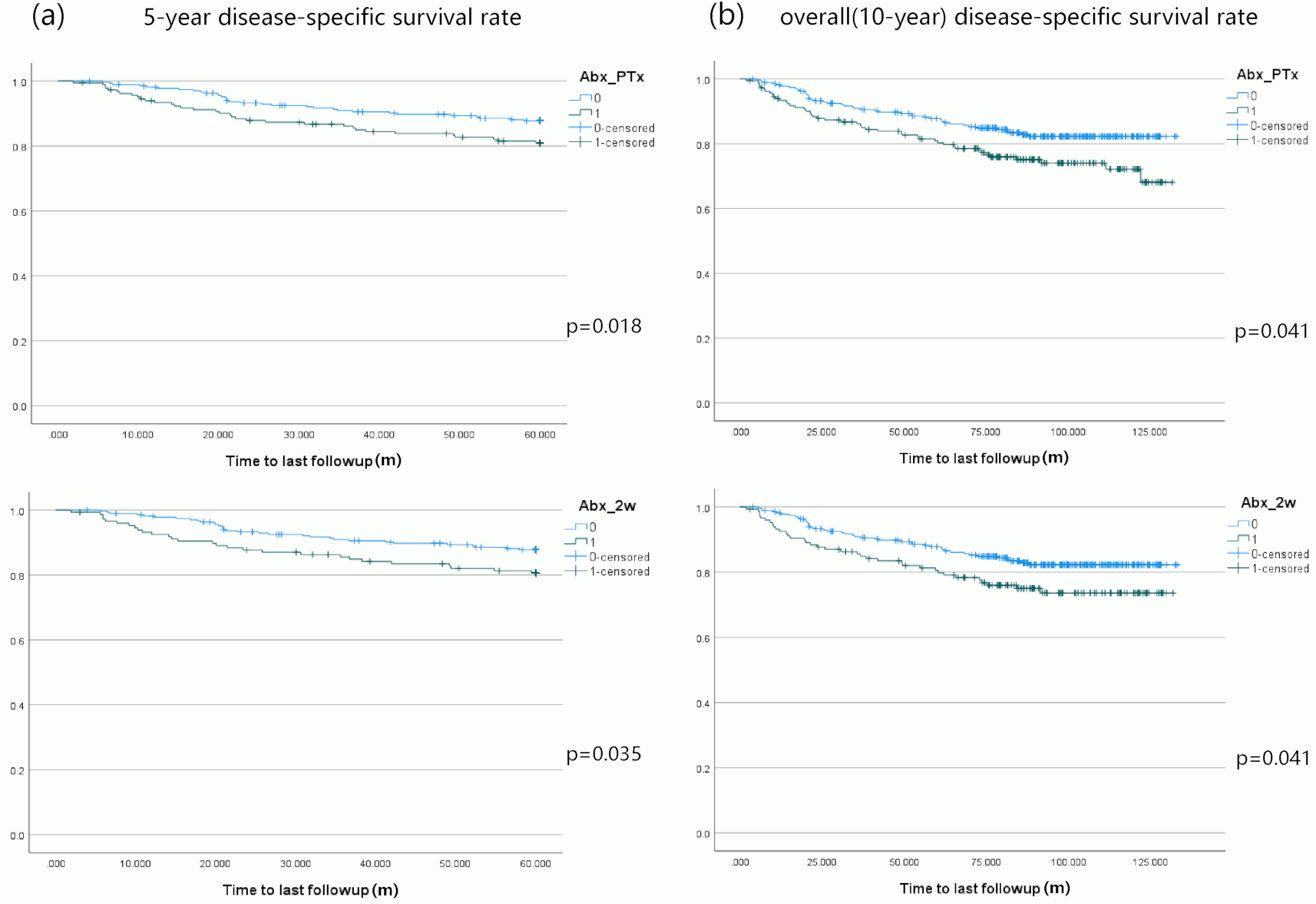
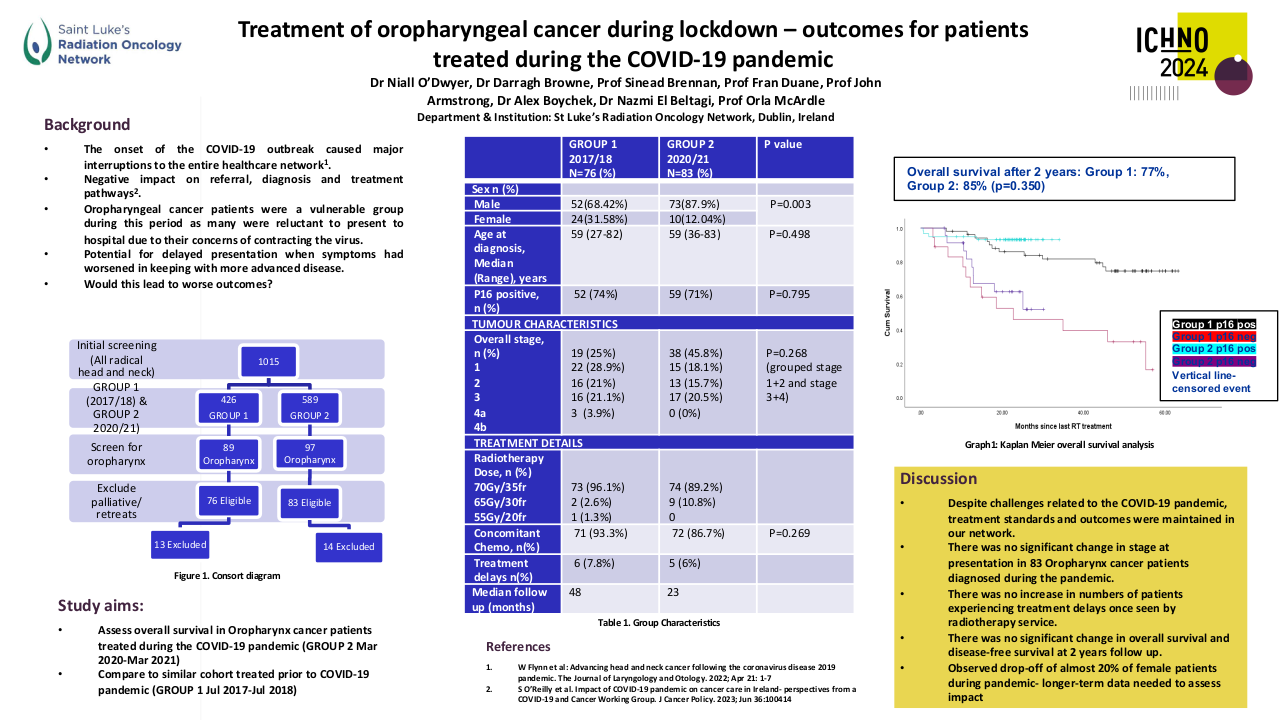
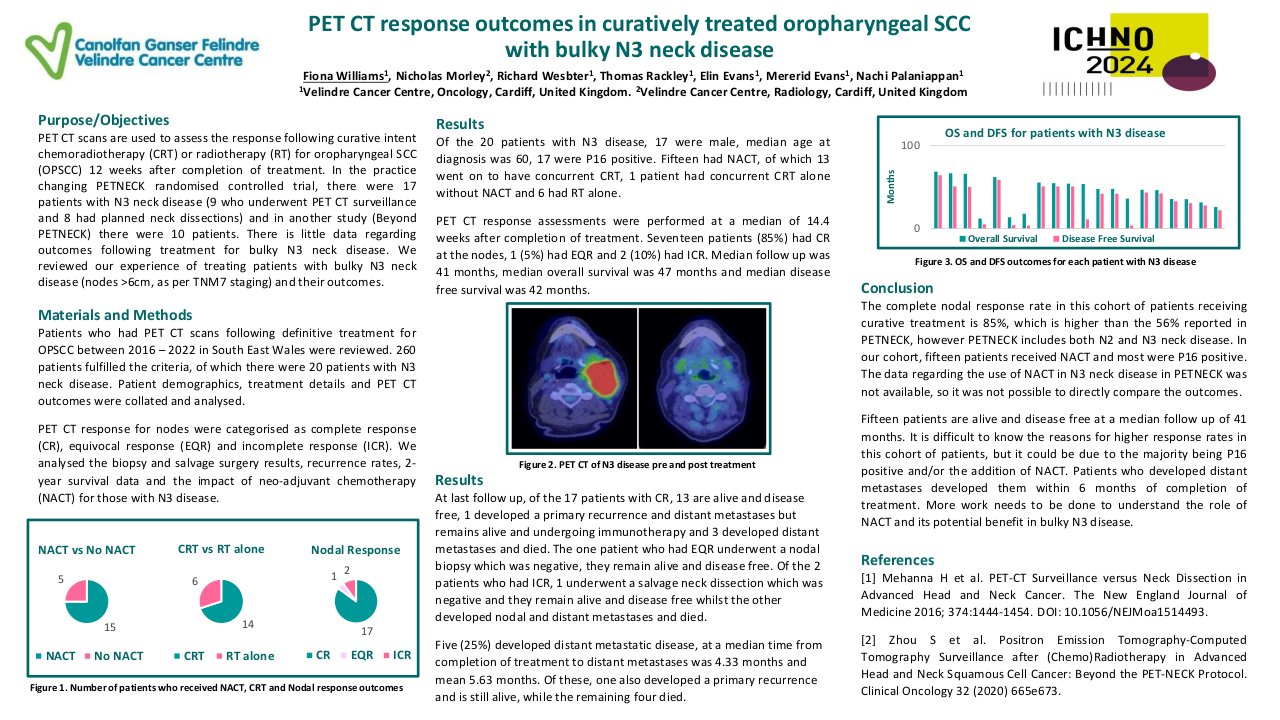
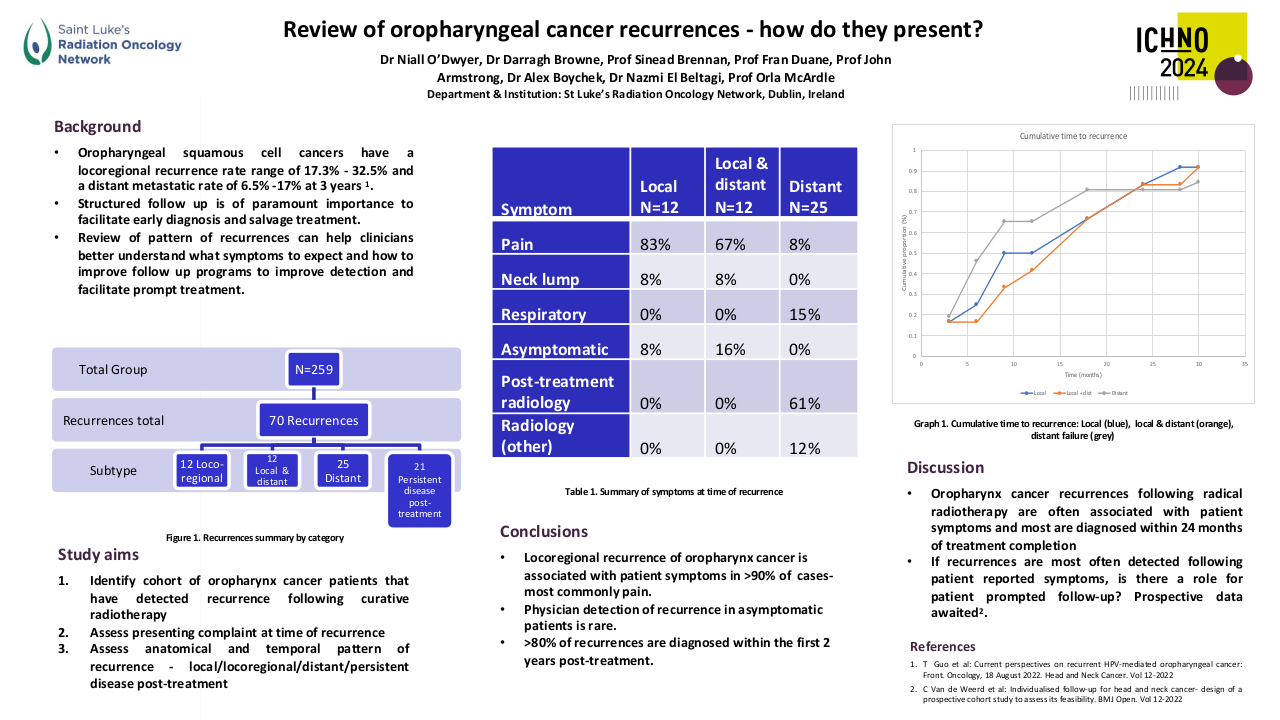
A total of 455 patients with primary NPC were included in the study. The cohort consisted of predominantly males (75.6%). Patients who had advanced tumor stage (p=0.019), received NC (p=0.008) or CCRT (p=0.002), were more likely to be prescribed antibiotics. On univariate analysis smoking (p=0.02), advanced stage of disease (p=0.007), lymph node metastasis (p= 0.004), NC administration (P<0.001) and antibiotic use was associated with a poorer year DSS in NPC patients. Antibiotic use around primary NPC treatment (5-year: HR 1.644 95%CI [1.015, 2.665], 10-year: 1.640 [1.085, 2.480]), within 2 weeks (5-year: 1.685 [1.015, 2.799], 10-year: 1.600 [1.030, 2.486]) or 1 week (5-year: 1.811 [1.073, 3.055]), were associated with significantly worse DSS in NPC patients, Figure 1 shows the Kaplan Meir curves. The negative elements included using specific antibiotics (5-year: 2.53 [1.331, 4.807], 10-year: 2.215 [1.236, 3.969]) and oral administration as well (5-year: 1.731 [1.069, 2.805], 10-year: 1.647 [1.087, 2.497]). Multivariate analysis showed a trend towards worse DSS at 5 years with antibiotic use at 1 week around therapy (P=0.054).
Conclusion
In NPC, antibiotics use around primary treatment, regardless of stage, had a poorer prognosis, especially disease-specific survival. In clinical practice, events leading to the prescription of antibiotics or the administration of antibiotics should be carefully considered in NPC patients treated for primary NPC.
1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80.2. Chang ET, Ye W, Zeng YX, Adami HO. The Evolving Epidemiology of Nasopharyngeal Carcinoma. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1035-47.3. Barnes L, Eveson J, Reichart P, Sidransky D. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. 2005.4. Bossi P, Chan AT, Licitra L, Trama A, Orlandi E, Hui EP, et al. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up(†). Ann Oncol. 2021;32(4):452-65.5. Tang LL, Chen YP, Chen CB, Chen MY, Chen NY, Chen XZ, et al. The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun (Lond). 2021;41(11):1195-227.6. Chen YP, Ismaila N, Chua MLK, Colevas AD, Haddad R, Huang SH, et al. Chemotherapy in Combination With Radiotherapy for Definitive-Intent Treatment of Stage II-IVA Nasopharyngeal Carcinoma: CSCO and ASCO Guideline. J Clin Oncol. 2021;39(7):840-59.7. Xia C, Jiang C, Li W, Wei J, Hong H, Li J, et al. A Phase II Randomized Clinical Trial and Mechanistic Studies Using Improved Probiotics to Prevent Oral Mucositis Induced by Concurrent Radiotherapy and Chemotherapy in Nasopharyngeal Carcinoma. Front Immunol. 2021;12:618150.8. Hou J, Zheng H, Li P, Liu H, Zhou H, Yang X. Distinct shifts in the oral microbiota are associated with the progression and aggravation of mucositis during radiotherapy. Radiother Oncol. 2018;129(1):44-51.9. Jiang C, Wang H, Xia C, Dong Q, Chen E, Qiu Y, et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer. 2019;125(7):1081-90.10. Nenclares P, Bhide SA, Sandoval-Insausti H, Pialat P, Gunn L, Melcher A, et al. Impact of antibiotic use during curative treatment of locally advanced head and neck cancers with chemotherapy and radiotherapy. Eur J Cancer. 2020;131:9-15.11. Köstler WJ, Hejna M, Wenzel C, Zielinski CC. Oral mucositis complicating chemotherapy and/or radiotherapy: options for prevention and treatment. CA Cancer J Clin. 2001;51(5):290-315.12. Taplitz RA, Kennedy EB, Bow EJ, Crews J, Gleason C, Hawley DK, et al. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J Clin Oncol. 2018;36(30):3043-54.13. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509-20.14. Yin J, Xie J, Lin J, Weng C, Lu S, Xu P, et al. Evaluation of the efficacy of the anti-ulcer oral mucosal protective agent RADoralex® in the prevention and treatment of radiation-induced oral mucosal reactions induced during treatment of nasopharyngeal carcinoma. Cancer Biol Ther. 2022;23(1):27-33.15. Maxwell MB, Maher KE. Chemotherapy-induced myelosuppression. Semin Oncol Nurs. 1992;8(2):113-23.16. Saloustros E, Tryfonidis K, Georgoulias V. Prophylactic and therapeutic strategies in chemotherapy-induced neutropenia. Expert Opin Pharmacother. 2011;12(6):851-63.17. Zhou S, Huang H, Chen Q, Tan KS, Zhu Z, Peng Y, et al. Long-term defects of nasal epithelium barrier functions in patients with nasopharyngeal carcinoma post chemo-radiotherapy. Radiother Oncol. 2020;148:116-25.18. Bonomi M, Batt K. Supportive Management of Mucositis and Metabolic Derangements in Head and Neck Cancer Patients. Cancers (Basel). 2015;7(3):1743-57.19. Anderson CM, Lee CM, Saunders DP, Curtis A, Dunlap N, Nangia C, et al. Phase IIb, Randomized, Double-Blind Trial of GC4419 Versus Placebo to Reduce Severe Oral Mucositis Due to Concurrent Radiotherapy and Cisplatin For Head and Neck Cancer. J Clin Oncol. 2019;37(34):3256-65.20. Berger K, Schopohl D, Bollig A, Strobach D, Rieger C, Rublee D, et al. Burden of Oral Mucositis: A Systematic Review and Implications for Future Research. Oncol Res Treat. 2018;41(6):399-405.21. Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17(5):271-85.22. Zhu XX, Yang XJ, Chao YL, Zheng HM, Sheng HF, Liu HY, et al. The Potential Effect of Oral Microbiota in the Prediction of Mucositis During Radiotherapy for Nasopharyngeal Carcinoma. EBioMedicine. 2017;18:23-31.23. Reyes-Gibby CC, Wang J, Zhang L, Peterson CB, Do KA, Jenq RR, et al. Oral microbiome and onset of oral mucositis in patients with squamous cell carcinoma of the head and neck. Cancer. 2020;126(23):5124-36.24. Vesty A, Gear K, Biswas K, Mackenzie BW, Taylor MW, Douglas RG. Oral microbial influences on oral mucositis during radiotherapy treatment of head and neck cancer. Support Care Cancer. 2020;28(6):2683-91.25. Gunn GB, Mendoza TR, Garden AS, Wang XS, Shi Q, Morrison WH, et al. Minocycline for symptom reduction during radiation therapy for head and neck cancer: a randomized clinical trial. Support Care Cancer. 2020;28(1):261-9.26. Duan H, Yu L, Tian F, Zhai Q, Fan L, Chen W. Antibiotic-induced gut dysbiosis and barrier disruption and the potential protective strategies. Crit Rev Food Sci Nutr. 2022;62(6):1427-52.27. Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535(7610):65-74.28. Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity. 2010;33(5):736-51.29. Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232-6.30. Zhou CB, Zhou YL, Fang JY. Gut Microbiota in Cancer Immune Response and Immunotherapy. Trends Cancer. 2021;7(7):647-60.31. Fessler J, Matson V, Gajewski TF. Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer. 2019;7(1):108.32. Gorjifard S, Goldszmid RS. Microbiota-myeloid cell crosstalk beyond the gut. J Leukoc Biol. 2016;100(5):865-79.33. Uribe-Herranz M, Rafail S, Beghi S, Gil-de-Gómez L, Verginadis I, Bittinger K, et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J Clin Invest. 2020;130(1):466-79.34. Yang K, Hou Y, Zhang Y, Liang H, Sharma A, Zheng W, et al. Suppression of local type I interferon by gut microbiota-derived butyrate impairs antitumor effects of ionizing radiation. J Exp Med. 2021;218(3).35. Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity. 2016;45(4):931-43.36. Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467(7318):967-71.37. Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971-6.38. Gehrke T, Hackenberg S, Polat B, Wohlleben G, Hagen R, Kleinsasser N, et al. Combination of salinomycin and radiation effectively eliminates head and neck squamous cell carcinoma cells in vitro. Oncol Rep. 2018;39(4):1991-8.39. Aydemir L, Iplik ES, Ertugrul B, Kasarci G, Atas MN, Ulusan M, et al. Levofloxacin might be safe to use for OSCC patients. Med Oncol. 2021;38(8):87.40. Dong J, Li Y, Xiao H, Zhang S, Wang B, Wang H, et al. Oral microbiota affects the efficacy and prognosis of radiotherapy for colorectal cancer in mouse models. Cell Rep. 2021;37(4):109886.41. Tian X, Mei T, Yu M, Li Y, Ao R, Gong Y. The impact of antibiotic selection and interval time among advanced non-small cell lung cancer patients receiving prior antibacterial treatment and first-line chemotherapy. Cancer Med. 2022;11(24):4849-64.42. Chambers LM, Kuznicki M, Yao M, Chichura A, Gruner M, Reizes O, et al. Impact of antibiotic treatment during platinum chemotherapy on survival and recurrence in women with advanced epithelial ovarian cancer. Gynecol Oncol. 2020;159(3):699-705.43. Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. JAMA Oncol. 2019;5(12):1774-8.44. Cortellini A, Di Maio M, Nigro O, Leonetti A, Cortinovis DL, Aerts JG, et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J Immunother Cancer. 2021;9(4).