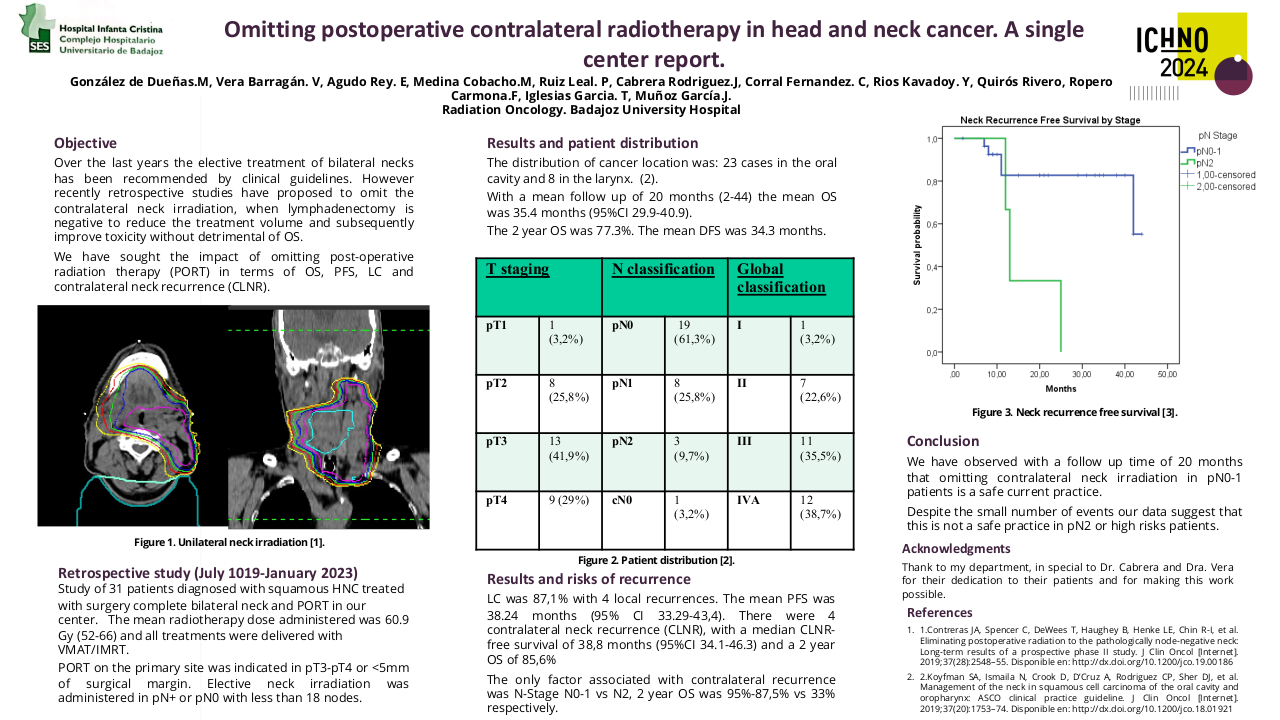
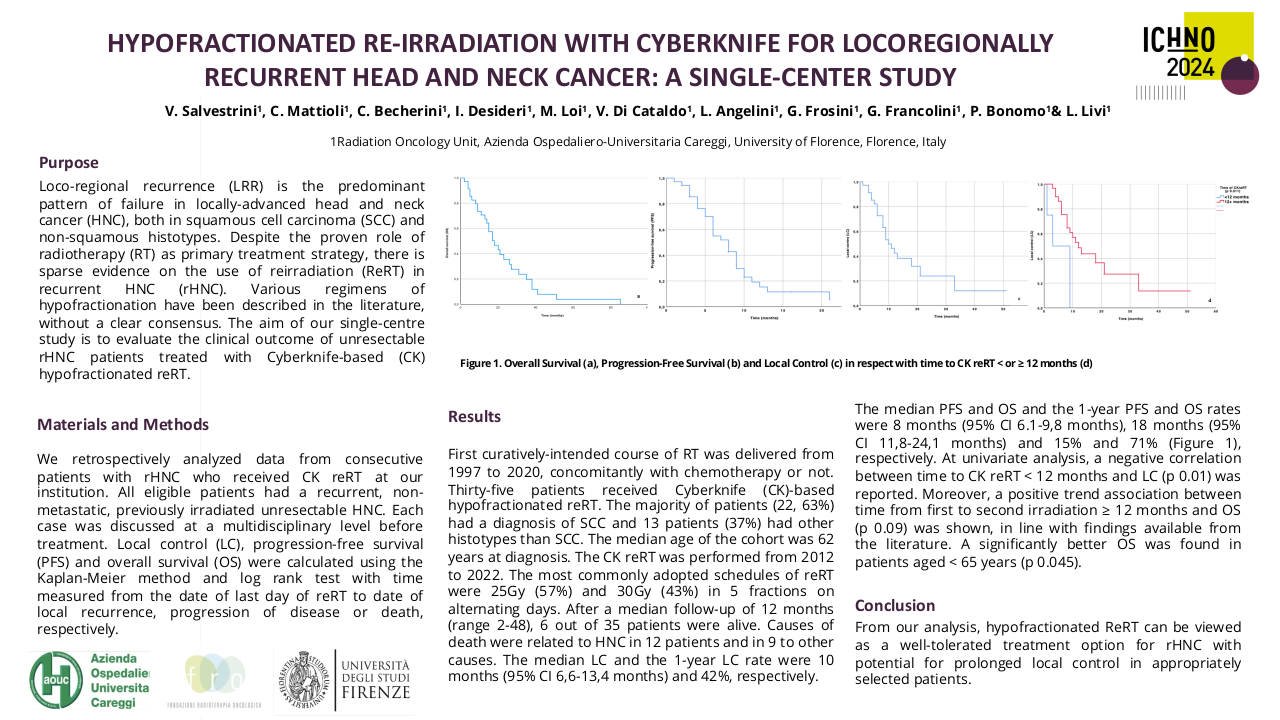
Long term outcomes of the FiGaRO trial: 18F-FDG-PET in Guided Dose-Painting with Intensity Modulated Radiotherapy in Oropharyngeal Tumours
Purpose/Objective
The FiGaRO trial assessed the feasibility and safety of using an FDG-PET-based dose-painting technique to deliver a radiotherapy (RT) boost to the FDG-avid primary tumour in patients with locally advanced high-to-intermediate risk oropharyngeal cancer. Patients were followed up within the study for 2 years. Patients treated at Guy’s and St Thomas’ hospital subsequently continued with standard clinical follow-up for a minimum of 5 years. The initial survival and toxicity data for all enrolled patients has previously been reported. The purpose of this further evaluation was to report the long-term survival and toxicity outcomes for patients treated within the FiGaRO trial at Guy’s and St Thomas’ Hospital.
Material/Methods
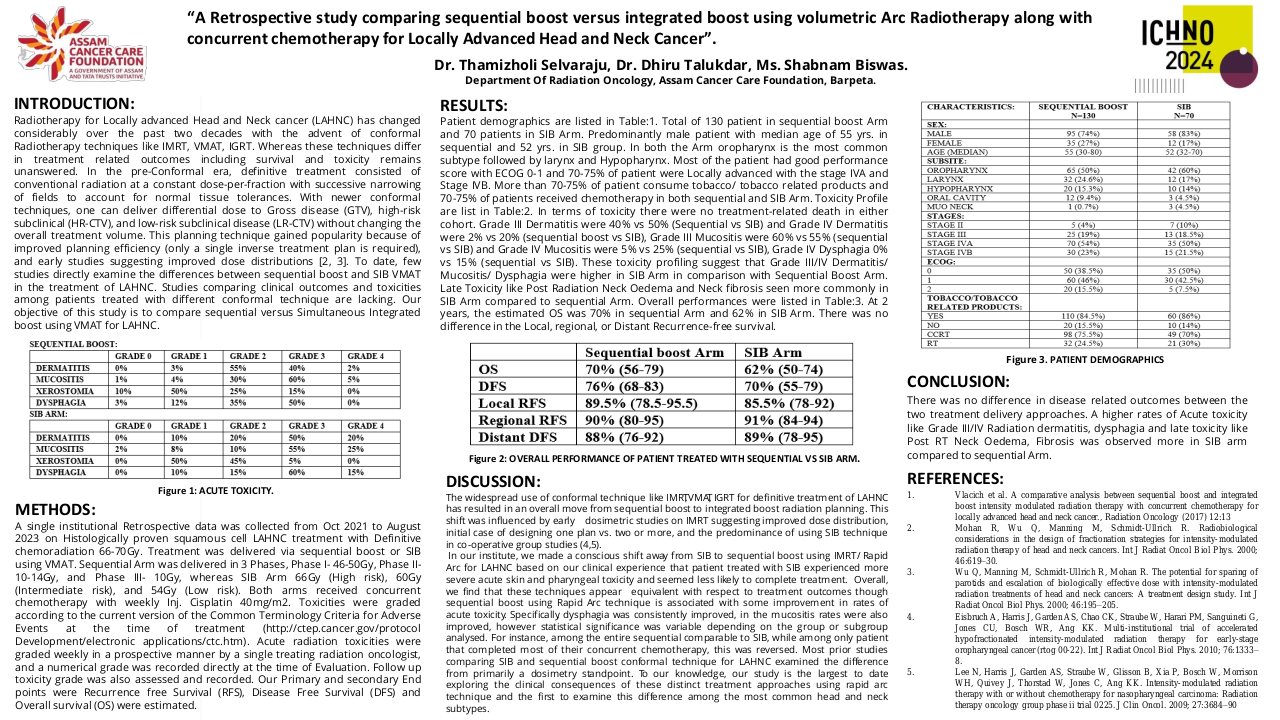
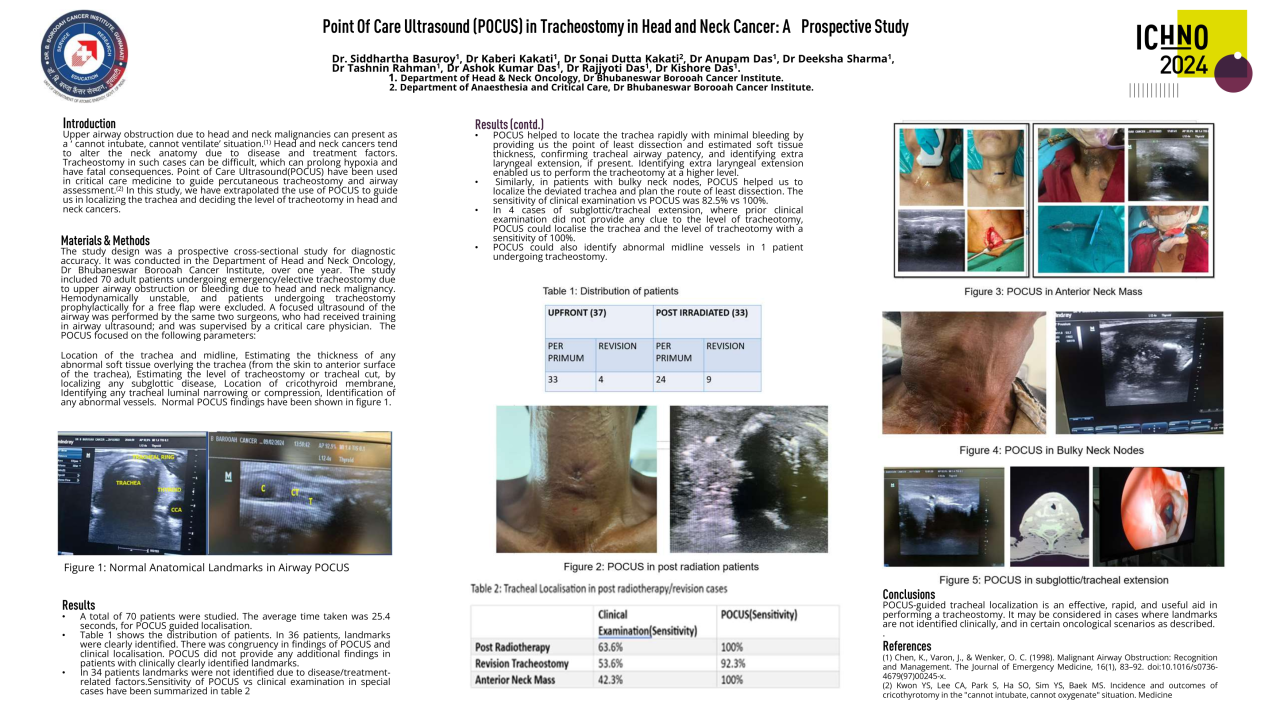
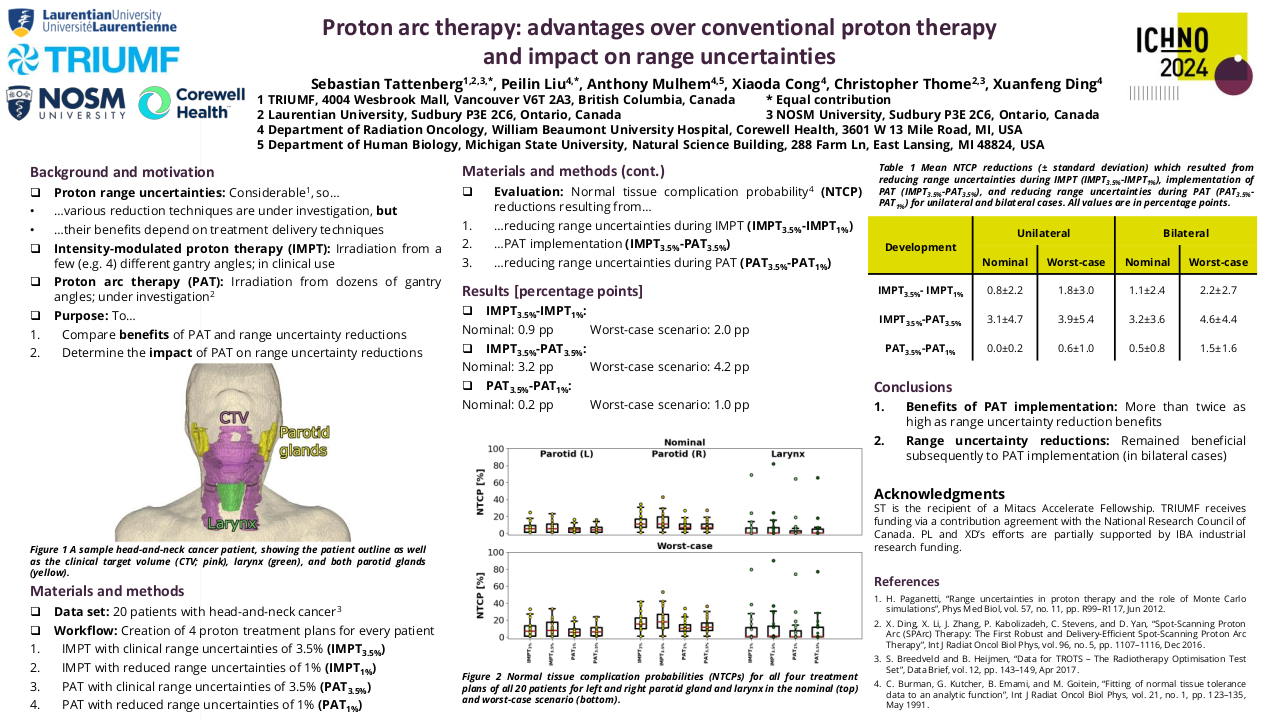
This study received local institutional approval as a service evaluation. Between April 2014 and October 2017 nineteen patients were treated within the study. Patients underwent a planning 18FDG-PET-CT scan (with contrast enhancement), immobilised in the treatment position, after one cycle of platinum-based induction chemotherapy. The volume of persistent FDG-avidity in the primary tumour, seen after 1 cycle of induction chemo, was escalated to 71.5 Gy in 30 fractions. The dose-escalation was delivered using a simultaneous integrated boost Intensity Modulated RT (SIB-IMRT) technique. Radiotherapy was delivered with concomitant cisplatin following 2 cycles of induction chemotherapy. On completion of trial-specific follow-up, patients were followed up annually until at least 5 years post-completion of radiotherapy. Late toxicity was assessed using Radiation Therapy Oncology Group (RTOG) and European Organization for Research and Treatment of Cancer (EORTC), and Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 toxicity scales.
Results
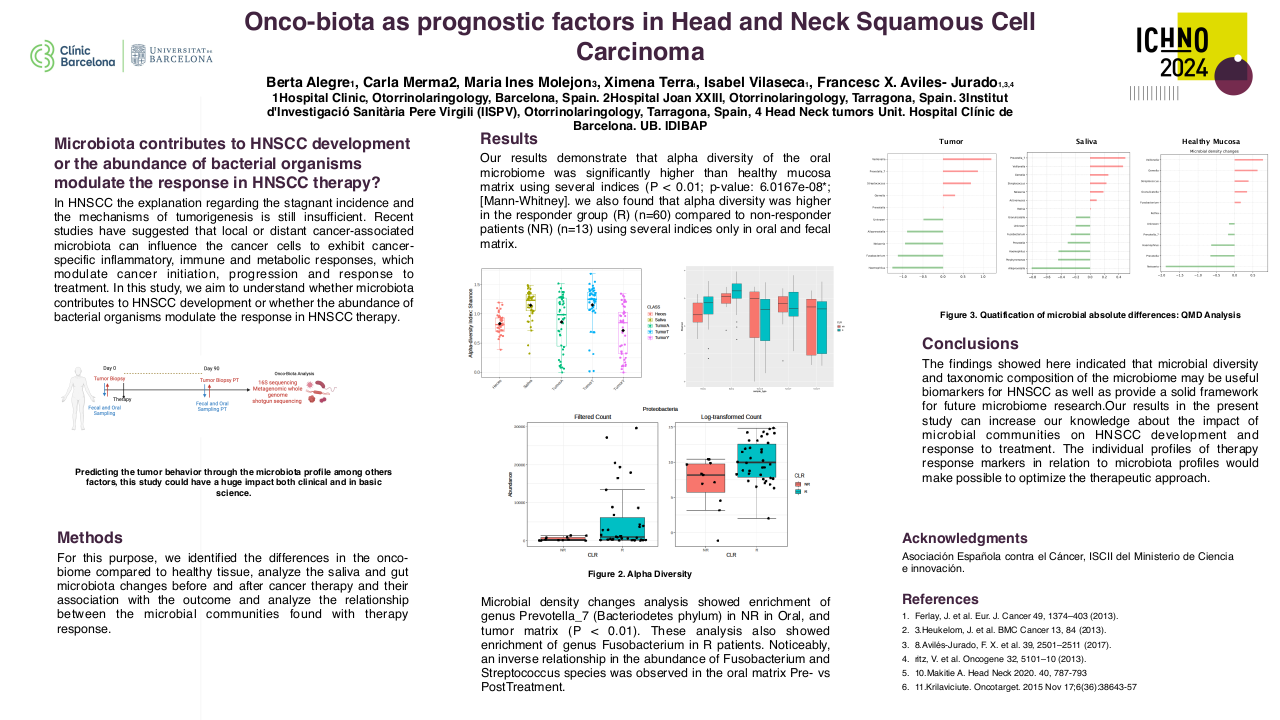
The median follow-up for living patients treated within the study was 68 months (range 61-101 months). All patients completed RT as prescribed. A median RT dose of 69.9 Gy (range 68.7-70.7) was delivered to ≥95% of the GTV-PET. Eleven patients had T4 primary tumours, three patients had a T3 tumour, and five patients had T2 tumours. All patients had nodal staging N2b or above.
During follow-up there were three local recurrences which all occurred within 12 months of treatment completion. Local control at 5-years was 83.9%. Five-year overall and disease-specific survival was 73.7% and 78.9%, respectively. The 5-year disease-specific survival was superior for intermediate-risk versus high-risk patients: 83.3% versus 71.4%, although this did not reach statistical significance.
Within this cohort there were no cases of persistent grade 3 or more mucosal toxicity, or persistent feeding tube use at 2 or more years post-treatment. Late grade 3 toxicity was limited to CTCAE grade 3 hearing loss (n=2) at 5 years, and RTOG grade 3 osteoradionecrosis of the jaw at 2- and 5 years (n=1). There were no further cases of grade 3 or more toxicity. The predominant toxicities at 2 years were; CTCAE grade 2 dysphagia (n=3) and xerostomia (n=5), and RTOG grade 2 mucosal toxicity (n=3). The predominant toxicities at 5 years were; CTCAE grade 2 dysphagia and xerostomia (n=3, respectively), and RTOG grade 2 mucosal toxicity (n=3).
Conclusion
The long-term outcomes suggest acceptable late toxicity rates and promising local control and disease-specific survival rates for patient treated within the FiGaRO trial. The escalation dose used in FiGaRO may be a suitable dose for further adaptive dose-escalation radiotherapy studies.
Michaelidou A, Adjogatse D, Suh Y, Pike L, Thomas C, Woodley O, Rackely T, Palaniappan N, Jayaprakasam V, Sanchez-Nieto B, Evans M. 18F-FDG-PET in guided dose-painting with intensity modulated radiotherapy in oropharyngeal tumours: A phase I study (FiGaRO). Radiotherapy and Oncology. 2021 Feb 1;155:261-8.