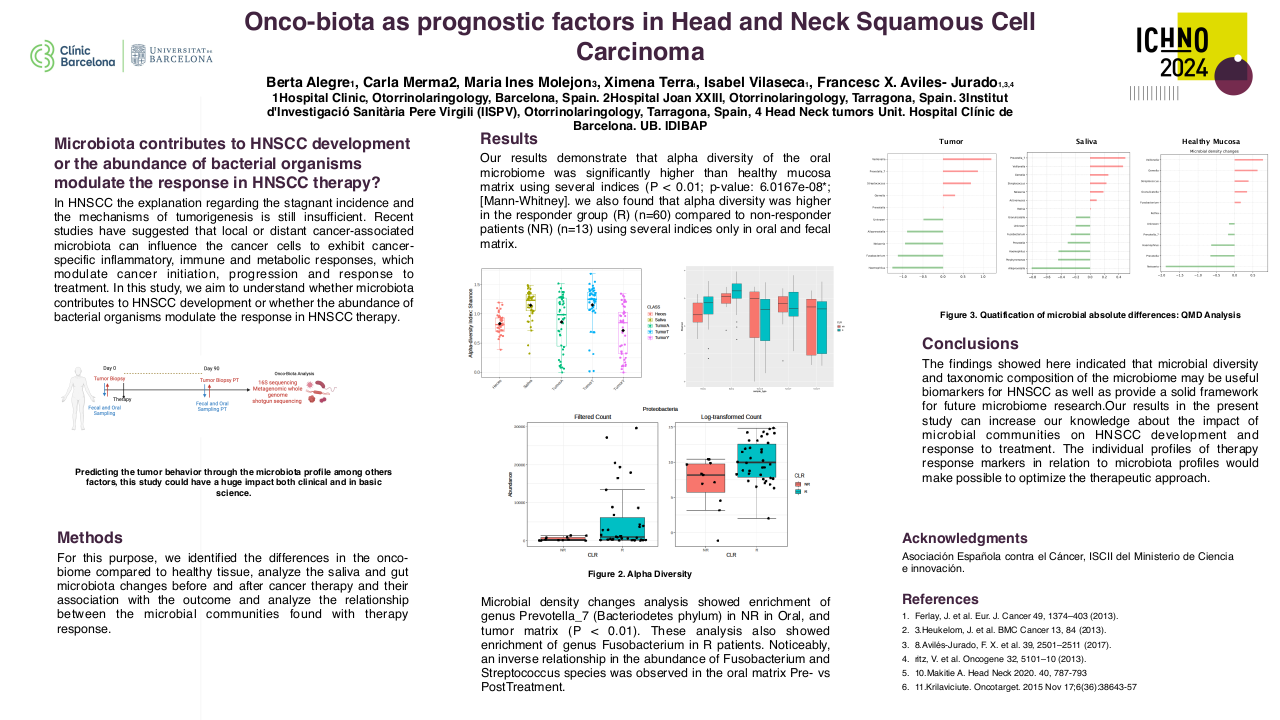
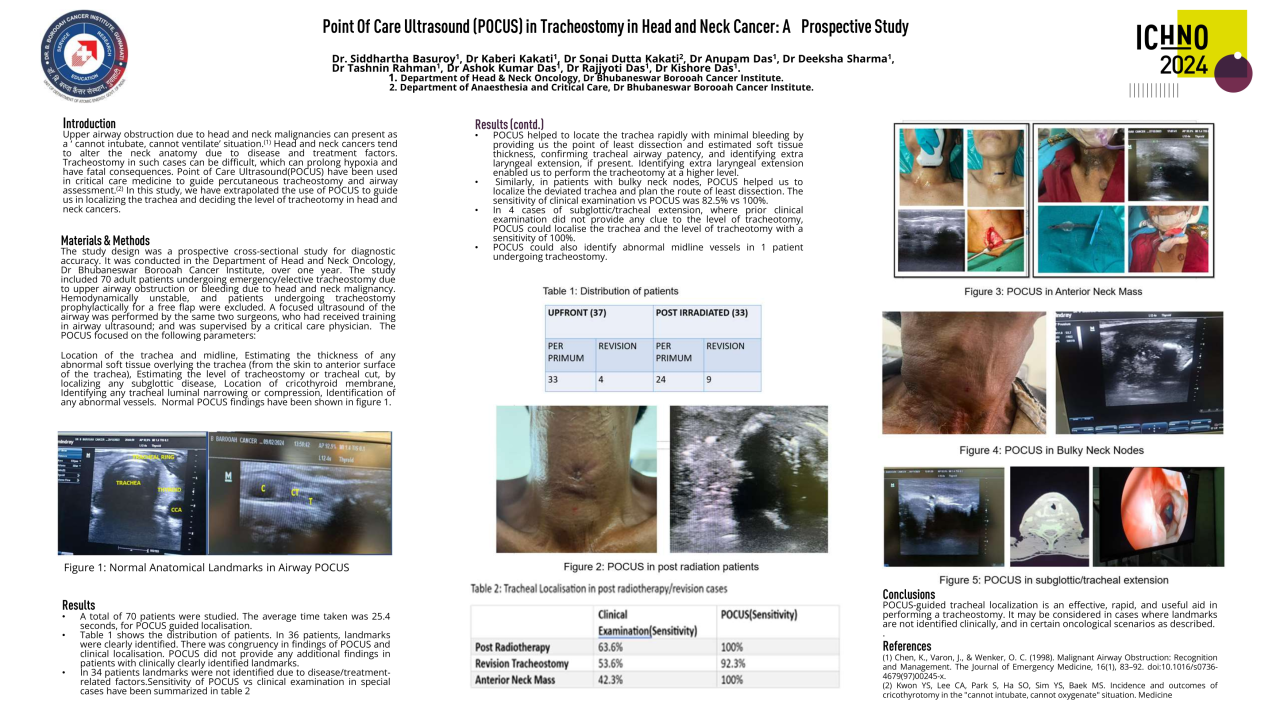
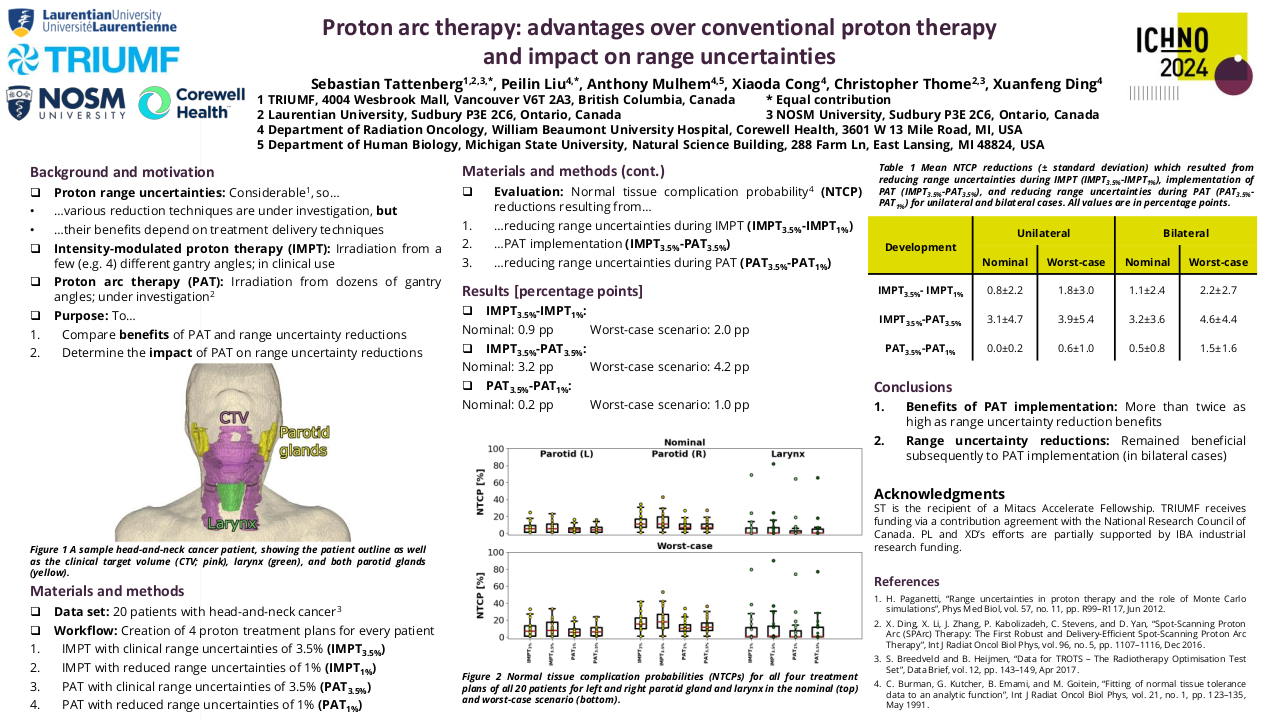
Proton arc therapy: advantages over conventional proton therapy and impact on range uncertainties
Purpose/Objective
Proton therapy for cancer reduces the integral radiation dose to the patient compared to conventional photon treatments.1 The number of proton therapy centers globally has been growing rapidly as a result, but uncertainties in the in vivo proton range remain a considerable hurdle.2,3 A variety of different approaches to reduce proton range uncertainties are therefore currently being pursued.4,5,6,7,8,9,10 The associated benefits have been quantified previously but depend on clinical practices, including the number of gantry angles from which the tumor is irradiated.11,12,13,14 For conventional proton therapy techniques like intensity-modulated proton therapy (IMPT), the target is irradiated from only a few directions, but proton arc therapy (PAT), for which the target is irradiated from hundreds of angles, may see clinical implementation by the time considerable range uncertainty reductions are achieved.15,16,17,18,19 It is therefore crucial to determine the impact of PAT implementation on the importance of range uncertainty reductions.
Material/Methods
Five head-and-neck cancer patients were randomly selected from The Radiotherapy Optimization Test Set (TROTS).20 For each patient, four different radiotherapy treatment plans were created in Version 6.0 of RayStation (RaySearch Laboratories, Stockholm, Sweden): an IMPT and a PAT treatment plan assuming current clinical range uncertainties of 3.5% (IMPT3.5% and PAT3.5%), and an IMPT and a PAT treatment plan assuming that range uncertainties can be reduced to 1% (IMPT1% and PAT1%). Treatment plans were evaluated with respect to target coverage and organ-at-risk (OAR) doses as well as normal tissue complication probabilities (NTCPs) resulting from organ irradiation during radiotherapy delivery. NTCPs were determined for both parotid glands (endpoint: parotid gland flow < 25% compared to pre-treatment after one year (grade 4 xerostomia)) and the larynx (endpoint: grade ≥ 2 larynx edema).21,22,23 In accordance with clinical practice, radiotherapy treatment planning assumed a patient setup uncertainty of 3 mm, and dose calculations applied a constant relative biological effectiveness (RBE) value of 1.1 to account for differences between proton and photon irradiation.12
Results
For all four types of treatment plans and all delineated organs, OAR doses are shown in Table 1. Values are given in the nominal delivery scenario (in which neither patient setup nor proton range errors occur) as well as in the worst-case scenario (in which errors in patient setup and/or the proton range do occur during treatment delivery) and constitute average values over all five patients. On average, implementation of PAT (IMPT3.5%-PAT3.5%) reduced NTCPs in the nominal and worst-case scenario by 2.51 percentage points (pp) and 3.96 pp, respectively. In comparison, reducing range uncertainties from 3.5% to 1% during continued use of IMPT (IMPT3.5%-IMPT1%) reduced evaluated NTCPs by 0.64 pp and 1.27 pp, respectively. Average benefits of range uncertainty reductions subsequently to PAT implementation (PAT3.5%- PAT1%) were 0.81 pp in the nominal and 1.78 pp in the worst-case scenario.
Table 1 Organ-at-risk doses for all four types of treatment plans averaged over all five patients. Nom and WC refer to the nominal and the worst-case delivery scenario, respectively. All values are in units of Gy(RBE) and concern the mean dose within the organ in question, with the exception of the brainstem and the spinal cord, for which the maximum value within the organ is given. SMG refers to the submandibular glands while other abbreviations indicate swallowing muscles.
Organ |
IMPT1% |
IMPT3.5% |
PAT1% |
PAT3.5% |
||||
| Nom |
WC |
Nom |
WC |
Nom |
WC |
Nom |
WC |
|
| Brainstem |
23.9 |
27.4 |
25.3 |
29.2 |
25.8 |
30.8 |
25.6 |
29.7 |
| Larynx |
27.1 |
30.4 |
27.3 |
30.7 |
23.3 |
26.1 |
24.1 |
27.3 |
| MCI |
18.8 |
21.6 |
19.2 |
22.4 |
14.5 |
17.2 |
15.8 |
19.6 |
| MCM |
54.9 |
57.6 |
55.2 |
58.1 |
49.2 |
52.9 |
49.7 |
53.9 |
| MCP |
9.2 |
11.7 |
9.6 |
12.4 |
7.0 |
9.1 |
7.7 |
10.3 |
| Oesophagus |
5.8 |
8.1 |
5.9 |
8.2 |
4.0 |
5.7 |
4.6 |
6.5 |
| Oral cavity |
24.0 |
27.3 |
25.0 |
28.8 |
22.0 |
24.7 |
23.4 |
26.8 |
| Parotid (L) |
16.3 |
20.2 |
17.0 |
21.2 |
14.8 |
18.1 |
15.6 |
19.7 |
| Parotid (R) |
18.7 |
23.6 |
19.4 |
24.5 |
16.2 |
20.3 |
17.4 |
22.0 |
| SCM |
51.0 |
55.4 |
51.9 |
56.0 |
47.3 |
51.1 |
48.4 |
52.9 |
| SMG (L) |
47.5 |
52.0 |
48.5 |
53.1 |
44.7 |
48.8 |
46.2 |
50.8 |
| SMG (R) |
57.7 |
59.9 |
57.9 |
60.1 |
56.3 |
58.3 |
56.7 |
59.4 |
| Spinal cord |
43.9 |
46.2 |
45.1 |
47.6 |
37.3 |
39.4 |
34.3 |
38.5 |
Conclusion
The average clinical benefit of implementing PAT was approximately three times higher than the benefit of a 3.5% to 1% range uncertainty reduction during continued use of IMPT. Reducing range uncertainties provided a similar clinical benefit for PAT and IMPT. Range uncertainty reductions are therefore expected to remain beneficial even when achieved subsequently to or in tandem with PAT implementation.
1. R. R. Wilson, “Radiological use of fast protons,” Radiology, vol. 47, no. 5, pp. 487–491, Nov 1946.2. “Particle therapy facilities in clinical operation (last update: (August 2023),” https://www.ptcog.site/index.php/facilities-in-operation-public, accessed: 2023-08-25.3. H. Paganetti, “Range uncertainties in proton therapy and the role of Monte Carlo simulations,” Phys Med Biol, vol. 57, no. 11, pp. R99–R117, Jun 2012.4. E. Baer, A. Lalonde, G. Royle, H.-M. Lu, and H. Bouchard, “The potential of dual-energy CT to reduce proton beam range uncertainties,” Med Phys, vol. 44, no. 6, pp. 2332–2344, Jun 2017.5. N. Peters, P. Wohlfahrt, C. Hofmann, C. Moehler, S. Menkel, M. Tschiche, M. Kraus, E. Troost, W. Enghardt, and C. Richter, “Reduction of clinical safety margins in proton therapy enabled by the clinical implementation of dual-energy CT for direct stopping-power prediction,” Radiother Oncol, vol. 166, pp. 71–78, Jan 2022.6. K. Parodi, H. Paganetti, H. Shih, S. Michaud, J. Loeffler, T. Delaney, N. Liebsch, J. Munzenrider, A. Fischman, A. Knopf, and T. Bortfeld, “Pa- tient study of in vivo verification of beam delivery and range, using positron emission tomography and computed tomography imaging after proton ther- apy,” Int J Radiat Oncol Biol Phys, vol. 68, no. 3, pp. 920–934, Jul 2007.7. C.-H. Min, C. Kim, M.-Y. Youn, and J.-W. Kim, “Prompt gamma mea- surements for locating the dose falloff region in the proton therapy,” Appl Phys Lett, vol. 89, no. 18, Nov 2006.8. J. M. Verburg and J. Seco, “Proton range verification through prompt gamma-ray spectroscopy,” Phys Med Biol, vol. 59, no. 23, pp. 7089–7106, Dec 2014.9. J. Tada, Y. Hayakawa, K. Hosono, and T. Inada, “Time resolved properties of acoustic pulses generated in water and in soft tissue by pulsed proton beam irradiation–a possibility of doses distribution monitoring in proton radiation therapy,” Med Phys, vol. 18, no. 6, pp. 1100–1104, Nov-Dec 1991.10. J. Schauer, H.-P. Wieser, Y. Huang, H. Ruser, J. Lascaud, M. Würl, A. Chmyrov, M. Vidal, J. Herault, V. Ntziachristos, W. Assmann, K. Par- odi, , and G. Dollinger, “Proton beam range verification by means of ionoa- coustic measurements at clinically relevant doses using a correlation-based evaluation,” Front Oncol, vol. 12, no. 925542, Nov 2022.11. S. van de Water, I. van Dam, D. Schaart, A. Al-Mamgani, B. Heijmen, and M. Hoogeman, “The price of robustness; impact of worst-case optimization on organ-at-risk dose and complication probability in intensity-modulated proton therapy for oropharyngeal cancer patients,” Radiother Oncol, vol. 120, no. 1, pp. 56–62, Jul 2016.12. D. Wagenaar, R. Kierkels, A. van der Schaaf, A. Meijers, D. Scandurra, N. Sijtsema, E. Korevaar, R. Steenbakkers, A. Knopf, J. Landendijk, and S. Both, “Head and neck IMPT probabilistic dose accumulation: Feasibility of a 2 mm setup uncertainty setting,” Radiother Oncol, vol. 154, pp. 45–52, Jan 2021.13. S. Tattenberg, T. Madden, B. Gorissen, T. Bortfeld, K. Parodi, and J. Ver- burg, “Proton range uncertainty reduction benefits for skull base tumors in terms of normal tissue complication probability (NTCP) and healthy tissue doses,” Med Phys, vol. 48, no. 9, pp. 5356–5366, Sep 2021.14. S. Tattenberg, T. Madden, T. Bortfeld, K. Parodi, and J. Verburg, “Range uncertainty reductions in proton therapy may lead to the feasibility of novel beam arrangements which improve organ-at-risk sparing,” Med Phys, vol. 49, no. 7, pp. 4693–4704, Jul 2022.15. A. Moreno, S. Frank, A. Garden, D. Rosenthal, C. Fuller, G. Gunn, J. Reddy, W. Morrison, T. Williamson, E. Holliday, J. Phan, and P. Blanchard, “Intensity Modulated Proton Therapy (IMPT) – The Future of IMRT for Head and Neck Cancer,” Oral Oncol, vol. 88, pp. 66-74, Jan 2020.16. X. Ding, X. Li, J. Zhang, P. Kabolizadeh, C. Stevens, and D. Yan, “Spot-Scanning Proton Arc (SPArc) Therapy: The First Robust and Delivery-Efficient Spot-Scanning Proton Arc Therapy,” Int J Radiat Oncol Biol Phys, vol. 96, no. 5, pp. 1107–1116, Dec 2016.17. B. de Jong, C. Battinelli, J. Free, D. Wagenaar, E. Engwall, G. Janssens, J. Langendijk, E. Korevaar, and S. Both, “Spot scanning proton arc therapy reduces toxicity in oropharyngeal cancer patients,” Med Phys, vol. 50, no. 3, pp. 1305–1317, Mar 2023.18. B. A. de Jong, E. W. Korevaar, A. Maring, C. I. Werkman, D. Scandurra, G. Janssens, S. Both, and J. A. Langendijk, “Proton arc therapy increases the benefit of proton therapy for oropharyngeal cancer patients in the model based clinic,” Radiother Oncol, vol. 184, p. 109670, Jul 2023.19. X. Li, G. Liu, G. Janssens, O. De Wilde, V. Bossier, X. Lerot, A. Pouppez, D. Yan, C. Stevens, P. Kabolizadeh, and X. Ding, “The first prototype of spot-scanning proton arc treatment delivery,” Radiother Oncol, vol. 137, p. 130-136, Aug 2019.20. S. Breedveld and B. Heijmen, “Data for TROTS – The Radiotherapy Optimisa- tion Test Set,” Data Brief, vol. 12, pp. 143–149, Apr 2017.21. C. Burman, G. Kutcher, B. Emami, and M. Goitein, “Fitting of normal tissue tolerance data to an analytic function,” Int J Radiat Oncol Biol Phys, vol. 21, no. 1, pp. 123–135, May 199122. T. Dijkema, C. Raaijmakers, R. Haken, J. Roesink, P. Braam, A. Houwel- ing, M. Moerland, A. Eisbruch, and C. Terhaard, “Parotid gland function after radiotherapy: the combined michigan and utrecht experience,” Int J Radiat Oncol Biol Phys, vol. 78, no. 2, pp. 449–453, Oct 2010.23. T. Rancati, M. Schwarz, A. Allen, F. Feng, A. Popovtzer, B. Mittal, and A. Eisbruch, “Radiation dose-volume effects in the larynx and pharynx,” Int J Radiat Oncol Biol Phys, vol. 76, no. 3S, pp. S64–S69, Mar 2010.