Nasopharyngeal cancer chemotherapy – before or after curative chemoradiation?
Purpose/Objective
Nasopharyngeal carcinomas (NPC) are endemic in southeast Asia and rare in Europe with an incidence of 0.07/100.000 persons. Five-year survival is about 50%. Diagnosis is provided by histological findings and staging classification is done according to AJCC. EBV DNA serum levels should be determined before and after local treatment, carry prognostic significance and can be used in the active surveillance of cancer survivors. The best method for serum determination of EBV DNA is still under discussion.
Advanced locoregional disease carries a greater risk of distant spread, highlighting the need of treatment intensification in higher risk patients. The best treatment plan is still under discussion: concurrent chemoradiation followed by adjuvant chemotherapy (ACT) or induction chemotherapy (ICT) followed by chemoradiation. ACT/ICT regimens should consist of two-/three-drug regimens, including a platinum agent and the best drug regimen is still under investigation. ACT carries great toxicity (50% require dose reductions, 60% complete treatment) and has a relatively low PFS and OS benefit. ICT is better tolerated, but may compromise cisplatin cumulative dose in concomitant chemoradiation and delay radiation start, possibly compromising the effectiveness of local treatment. Nonetheless, ICT improves PFS and OS when compared to chemoradiation alone, mostly because of better metastasis free survival (MFS), making this a promising strategy in properly selected high risk patients.
Most NPC trials were conducted in countries where NPC is endemic, primarily non-queratinizing and EBV-related. Data in non-endemic countries are lacking.
Our study aims to compare ACT and ICT in locally advanced and oligometastatic NPC patients treated in a European reference centre.
Material/Methods
Retrospective, observational study of patients with NPC diagnosis between January 2017 and September 2023 and disease stage III-IVb. Data were collected from patient records and included patient characteristics (gender, age, smoking history, ECOG performance status), tumor characteristics (T, N, staging, histology, EBV-status) and treatment characteristics (ACT, ICT, toxicities). PFS and OS were analyzed. Toxicity grading is according to CTCAE 5.0 and statistical analysis is descriptive.
Results
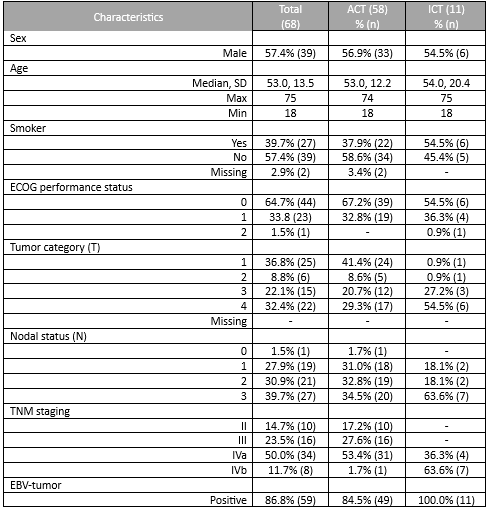
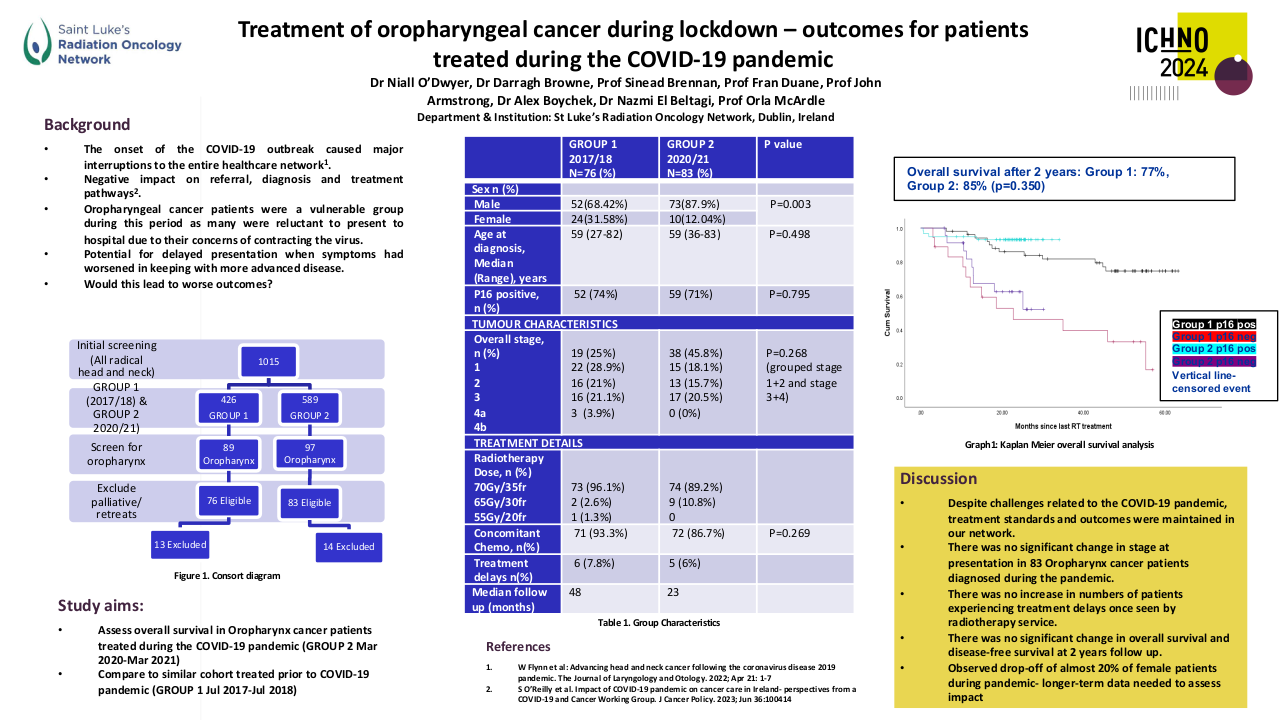
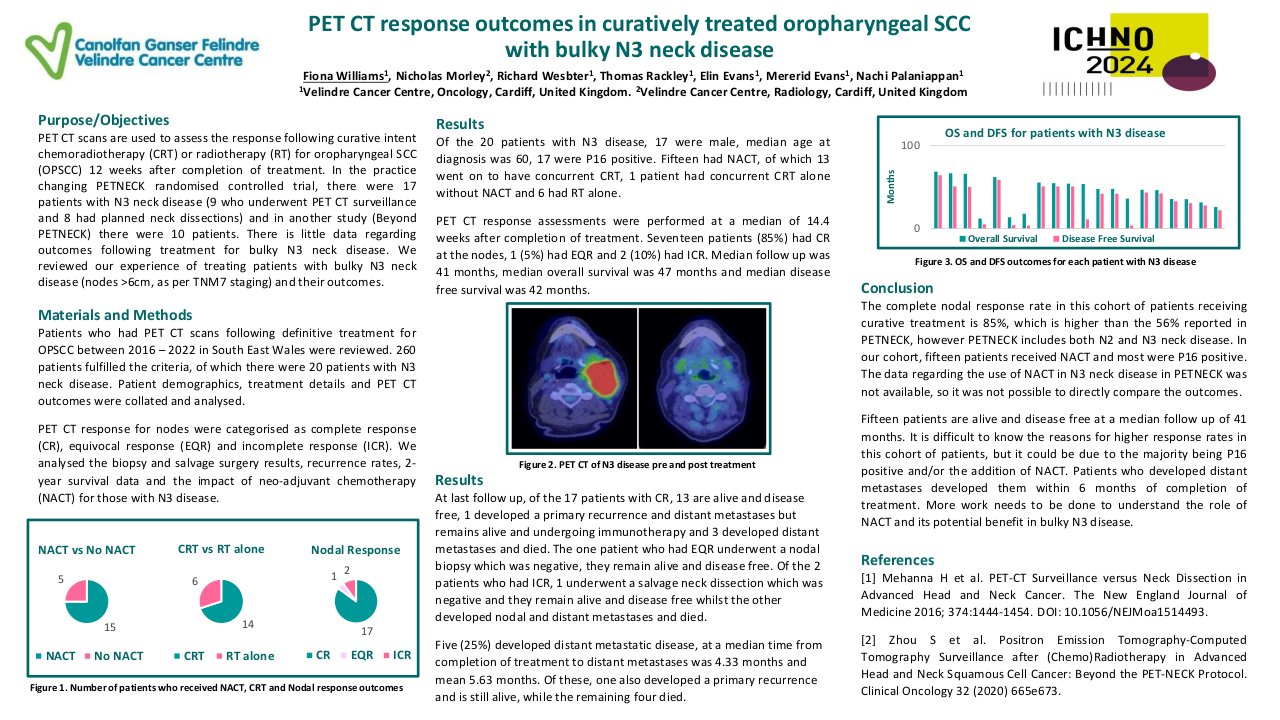
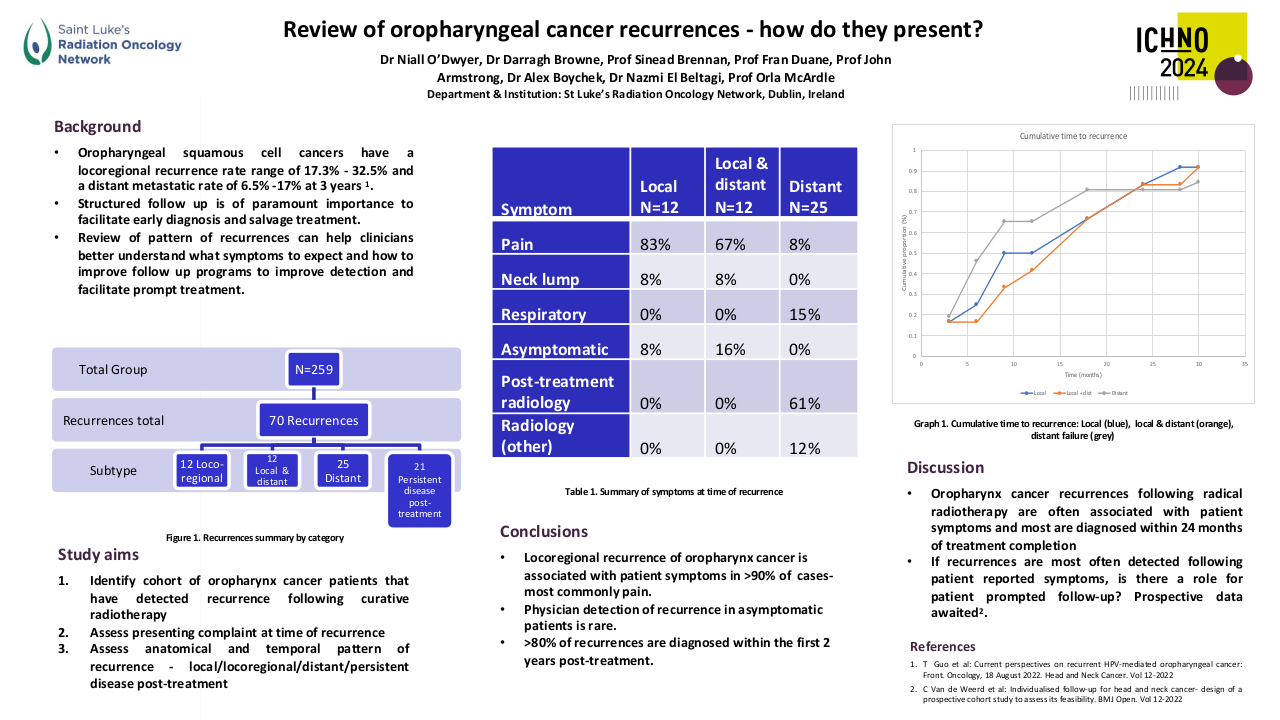
A total of 69 patients were included. Most patients were male (56.5%, n=39) with a median age of 53.0 years (18-75). Most patients had a good performance status (0 or 1 in 98.5%, n=68). Stage IVa was the most frequent initial staging (50.7%, n=35), with a majority of patients having T4 (33.3%, n=23) and N3 (39.1%, n=27) tumours. All tumours were undifferentiated queratinizing carcinomas and 86.9% (n=60) were EBV-positive. EBV DNA was rarely determined.
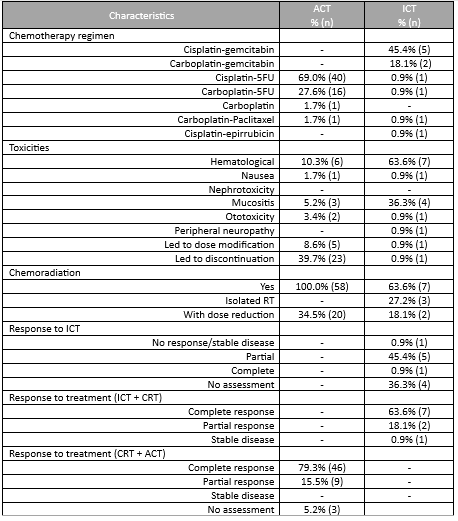
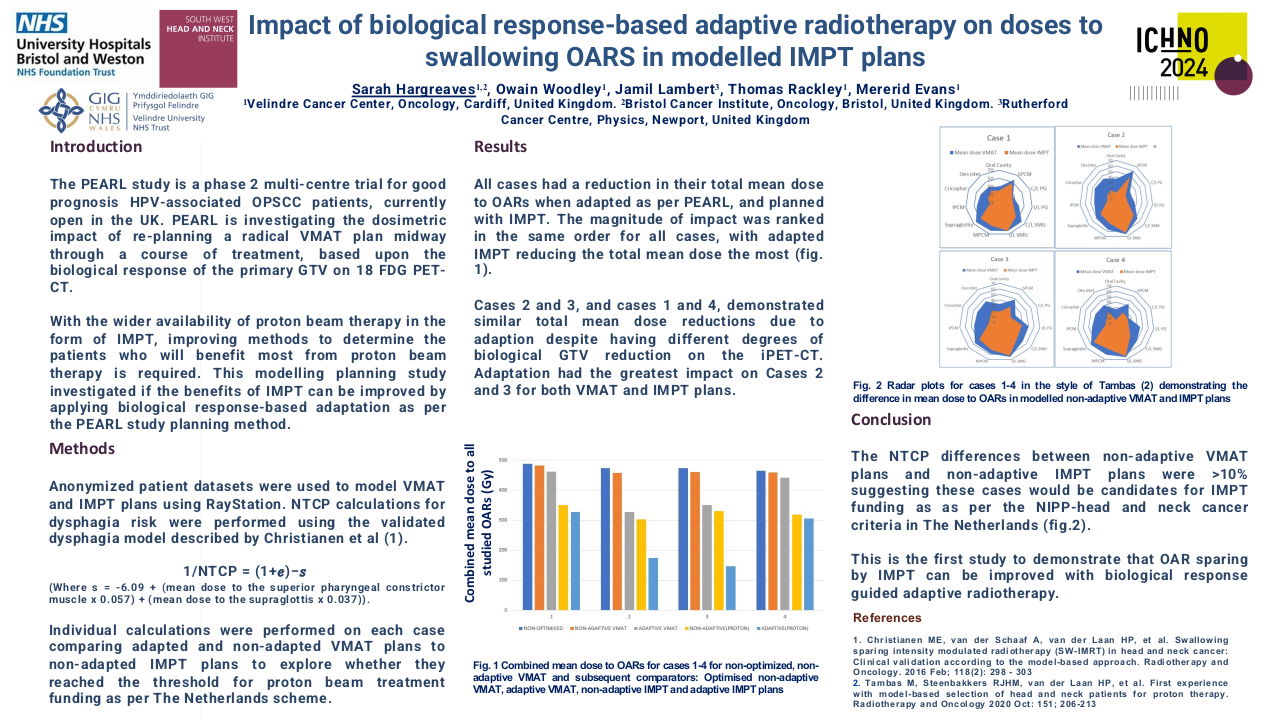
Until 2019 the most frequent treatment strategy was ACT with cisplatin-5FU (57.9%, n=40), after which ICT became more frequently used (15.9%, n=11), mostly with cisplatin-gemcitabin (45.4%, n=5). ICT had less acute toxicities that lead to treatment discontinuation (0.9% (n=1) versus 39.7% (n=23)). All patients completed radiotherapy after ICT, but in 4 patients optimal concomitant cisplatin dose (≥200mg/m2) was compromised. Both ICT and ACT showed good response rates, with a complete response in 63.6% versus 79.3%, respectively. Median follow up was 45 months in the ACT group (4-125) and 27 months (1-55) in the ICT group. PFS with ACT was 45.0 months (95%CI 34.8-55.2) and OS was 46.0 months (95%CI 38.5-53.4). The ICT group is small and has a short follow-up time; PFS and OS data will be determined in the future.
Conclusion
The best course of treatment of advanced NPC is still unclear - which patients and histologies benefit most from treatment intensification? What is the role of EBV DNA and what is the best method for its determination? Should ACT or ICT be preferred? Which chemotherapy regimen is better? Treatment center expertise and the ability to provide timely treatment may also play a role in treatment decisions.
Our study presents the experience of a European reference centre where ACT was the preferred treatment for a longer period of time and therefore has a significantly larger patient population with longer follow-up time. ACT seems to provide good patient outcomes, despite a worse toxicity profile and more related treatment discontinuations, but group comparison is not yet possible since our centre only recently changed strategies in managing advanced NPC patients, preferring ICT over ACT. ICT patients are still underrepresented in this analysis, with fewer patients and shorter follow-up time. Moreover, the ICT patient group has more advanced disease, including IVb oligometastatic disease (that was not included in the intensification ACT and ICT studies), making head-to-head comparisons difficult.
The management of advanced NPC is a clinical challenge and more data are needed to better select both patients and treatment strategies for treatment intensification, while taking into consideration the differences between endemic and non-endemic NPC.
1. Age Standardized (World) Incidence Rates, Nasopharynx, Males, All Ages.; 2020. https://gco.iarc.fr/today2. Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2021;32(4):452-465. doi:10.1016/j.annonc.2020.12.0073. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. The Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-04. Bossi P, Chan AT, Even C, Machiels JP. ESMO–EURACAN Clinical Practice Guideline update for nasopharyngeal carcinoma: adjuvant therapy and first-line treatment of recurrent/metastatic disease. Annals of Oncology. 2023;34(3):247-250. doi:10.1016/j.annonc.2022.11.0115. Haddad RI, Hicks WL, Hitchcock YJ, et al. NCCN Guidelines Version 2.2023 Head and Neck Cancers Continue NCCN Guidelines Panel Disclosures.; 2023. https://www.nccn.org/home/member-6. Ng WT, Chang ATY, Lee SWM, Sze HCK, Lee AWM. Chemotherapy for Nasopharyngeal Cancer: Neoadjuvant, Concomitant, and/or Adjuvant. Curr Treat Options Oncol. 2015;16(9). doi:10.1007/s11864-015-0361-57. Jiromaru R, Nakagawa T, Yasumatsu R. Advanced Nasopharyngeal Carcinoma: Current and Emerging Treatment Options. Cancer Manag Res. 2022;14:2681-2689. doi:10.2147/CMAR.S3414728. Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. New England Journal of Medicine. 2019;381(12):1124-1135. doi:10.1056/nejmoa19052879. Yuan C, Xu XH, Luo SW, et al. Which neoadjuvant chemotherapy regimen should be recommended for patients with advanced nasopharyngeal carcinoma? a network meta-analysis. Medicine (United States). 2018;97(34). doi:10.1097/MD.0000000000011978