Cisplatin Head & Neck Chemoradiotherapy: A Real-World Survival & Compliance Analysis of Weekly vs 3-Weekly Regimes
Purpose/Objective
Concurrent chemoradiotherapy with 100 mg/m2 cisplatin (Cis100) every 3 weeks has been the standard of care for locoregionally advanced head and neck squamous cell carcinomas (LAHNSCC) for many years (Grégoire). Full compliance with all three cycles is achieved in only around two thirds' cases (Szturz) and interest in alternative regimes has led some clinicians to adopt weekly cisplatin regime in the hope of improved toxicity, compliance and to ease capacity demands.
Low dose cisplatin chemotherapy regimens have been investigated in a number of studies. The use of weekly cisplatin at 40 mg/m2 (Cis40) is supported by a large, randomised phase III trial confirming weekly cisplatin is non-inferior to 3 weekly cisplatin, it was also better tolerated with less toxicity (Chatterjee). Additionally, evidence from the Japan Oncology Group found weekly Cis40 achieved comparable cumulative cisplatin dosing and was associated with a better overall survival (Kiyota).
Here we present a real-world retrospective analysis of 9 years of clinical data comparing weekly cisplatin versus 3-weekly cisplatin in both adjuvant and definitive settings for LAHNSCC. We aim to assess if there is a difference between regimens in terms of overall survival and assess compliance.
Material/Methods
Data was collected via the chemotherapy prescribing software, including all locally advanced head and neck patients registered for radical or adjuvant chemo-radiotherapy. Patients were treated in a single regional cancer centre between 02/09/2009 and 28/02/2018 with treatment was allocated according to clinician preference. Data was censored at date of death or at time of analysis with minimum 5.5yrs follow up.
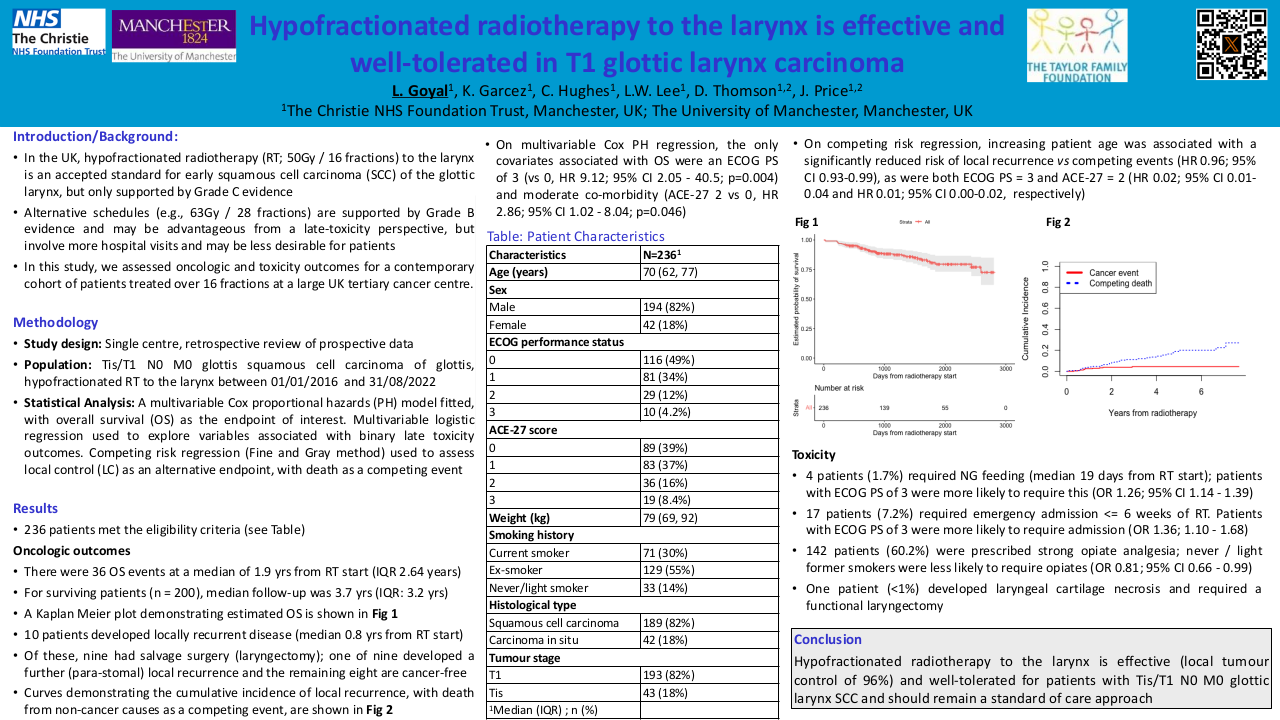
Results
Over the time period 335 patients were treated for locally advanced head and neck cancers with cisplatin + radiotherapy. Three patients within the data set had adenocarcinoma and were removed from analysis. Of 332 patients, 269 received standard dose Cis100 every 3 weeks, 63 patients received Cis40 weekly. The groups were equally balanced; approximately three quarters of patients were male and one quarter female in both regimens. In both groups the average age was 59 years, (range in in Cis100 group was 31-79years and Cis40 was 37-78years).
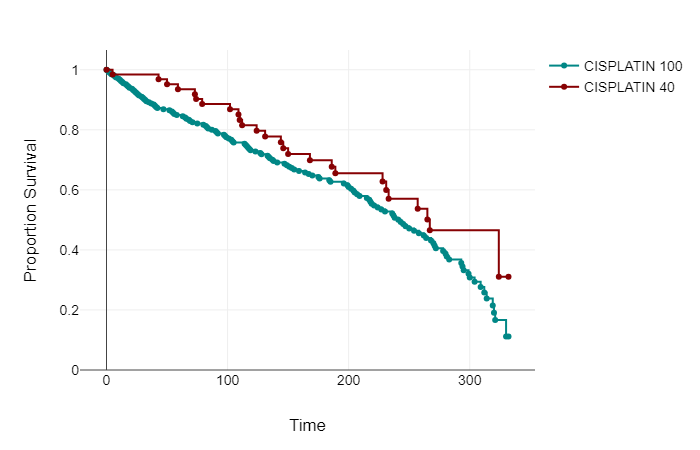
Event free survival was calculated from the time of first chemotherapy to death or censorship. In the whole cohort of Cis100 median EFS was 82 months, (95% CI 71.95 to 84.96 months). Within the Cis40 group median EFS was 74 months, (CI 53.17 to 74.61 months).
| Frequency |
Median |
Interquartile Range |
95% Confidence interval of Mean |
||
| mEFS from 1st Chemotherapy (months) |
CISPLATIN 100 |
269 |
82 |
100 |
71.95; 84.96 |
| CISPLATIN 40 |
63 |
74 |
66 |
53.17; 74.61 |
139 patients in Cis100 group had died at the time of evaluation, 26 within the Cis40 group.
A log-rank test was calculated to see if there was a difference between groups Cis100 and Cis40 in time to event occurrence (death). For the present data, the log-rank test showed that there is no significant difference between the groups in terms of the distribution of time until the event occurs, p=.086. See Graph 1.
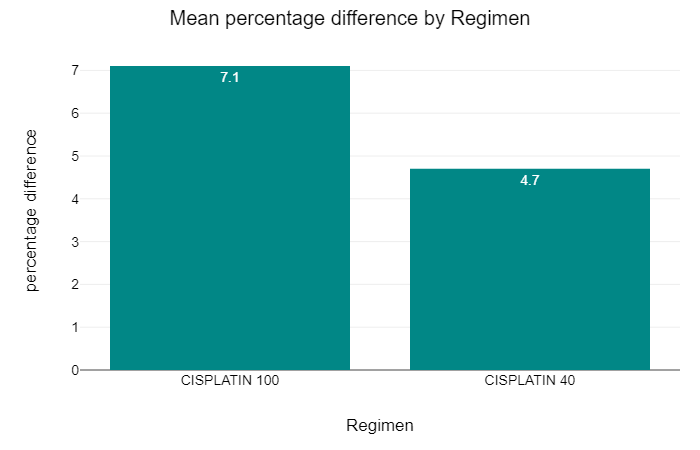
We wanted to assess the effect of chemotherapy dosing between the groups. Within the Cis100 group the average prescribed dose was 322mg of cisplatin with average received dose of 301mg, 13.4% of patients (36 of 269) received less than their prescribed dose. 50% or less of the prescribed dose was received by 11.2% (30 of 269).
In the Cis40 group the average prescribed dose was 321mg of cisplatin, with an average of 300mg received, 17.5% patients (11 of 63) received less than their prescribed dose of chemotherapy but only 1.6% (1 patient) received less than half of their prescribed dose.
The mean difference in chemotherapy prescribed to received was 7.1% and 4.7% in the Cis100 and Cis40 groups respectively. This showed a negative correlation which was not statistically significant. This is less than the previously documented reduction of dose within trial setting (Szturz).
The log-rank test was used to assess if a difference in OS existed between the two regimens taking into account the reduction of received chemotherapy. For the present data, there was no difference between the groups in terms of the distribution of time until the event occurs, p=.617.
Conclusion
This is a large real-world retrospective analysis suggesting no difference between Cis40 and Cis100 in terms of OS. There is a difference in percentage of chemotherapy successfully delivered between the regimens, but this does not translate into a difference in OS from our data.
Grégoire V, Lefebvre JL, Licitra L, Felip E; EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2010) 21(Suppl. 5):v184–6. doi: 10.1093/annonc/mdq185 Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V, et al. Weekly low-dose versus three-weekly high-dose cisplatin for concurrent chemoradiation in locoregionally advanced non-nasopharyngeal head and neck cancer: a systematic review and meta-analysis of aggregate data. Oncologist. (2017) 22:1056–66. doi: 10.1634/theoncologist.2017-0015Chatterjee, S, Kiyota, N, Vaish, R, et al. Weekly versus 3-weekly cisplatin along with radiotherapy for locoregionally advanced non-nasopharyngeal head and neck cancers: Is the equipoise in literature addressed yet? Head & Neck. 2023; 45(6): 1594-1603. doi:10.1002/hed.27365Kiyota N, Tahara M, Mizusawa J, et al. Weekly Cisplatin Plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. J Clin Oncol. 2022;40(18):1980-1990. doi:10.1200/JCO.21.01293