Induction TPF chemotherapy and its outcomes in head and neck cancer, a single centre experience
Purpose/Objective
Platinum, Docetaxel and Fluorouracil (5FU) (TPF) chemotherapy is a well-established regime used in locally advanced head and neck cancer patients to downstage disease prior to definitive treatment with chemo-radiotherapy. It has been shown to improved overall survival but can be associated with significant toxicity.1,2 We wished to examine our own experience in a large regional speciality oncology centre to review the survival and toxicity in our local patient cohort.
Material/Methods
A retrospective case review was conducted of all patients with head and neck cancers treated with platinum, Docetaxel and Fluorouracil (5FU) between January 2017 and December 2021. Data was collected on disease free survival (DFS), overall survival (OS), toxicity and further treatments.
Results
Eighty-four patients were identified; 82.1% (n=69) male and 17.8% (n=15) female, with a median age of 57.5 years. Most patients had primary disease in the oropharynx 85.7% (n=72) and of these 76.4% (n=55) were p16 positive. Using TNM8 staging, 33% were stage 4 (n=28) and 42% stage 3 (n=35). The median number of cycles of TPF given was 3.
Seventy-nine patients (94%) experienced side effects with toxicity of any grade; however most toxicity was low grade and only 22% (n=19) of patents required dose reduction. The most common forms of toxicity were fatigue (74.7%), nausea (44.3%), and diarrhoea (38%). In this cohort, 27.4% (n=23) of patients needed inpatient admission. The most common reason for admission was neutropenia and sepsis. There were two TPF toxicity related deaths from sepsis in this cohort and one further patient died from non-chemotherapy or cancer related causes prior to proceeding to definitive radiotherapy.
Eight-one patients proceeded to definitive radiotherapy, 70Gy in 35# over 7 weeks. Fifty-six patients received concurrent cisplatin (n=28 weekly, n=28 3-weekly), 17 carboplatin (n=8 weekly, n=9 weekly dosing regimens respectively), three received radiotherapy without chemotherapy and five patients received a historical platinum/5 Fluorouracil regime, 2 cycles at weeks 1 and 4.
Out of 81 patients, 16 were scanned with PET-CT prior to radiotherapy; 11 of these patients showed complete metabolic response (CMR), four showed partial metabolic response (PMR) and one showed stable disease. End of treatment scans were completed in 79 patients; 79.7% (n=63) were in CMR, 10.1% (n=8) PMR, disease progression in 8.9%(n=7), and stable disease in 1.3% (n=1). Outcome was not available in two patients who were lost to follow up.
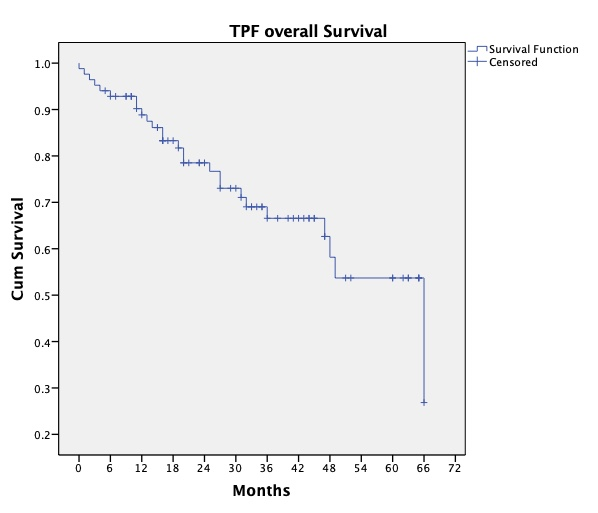
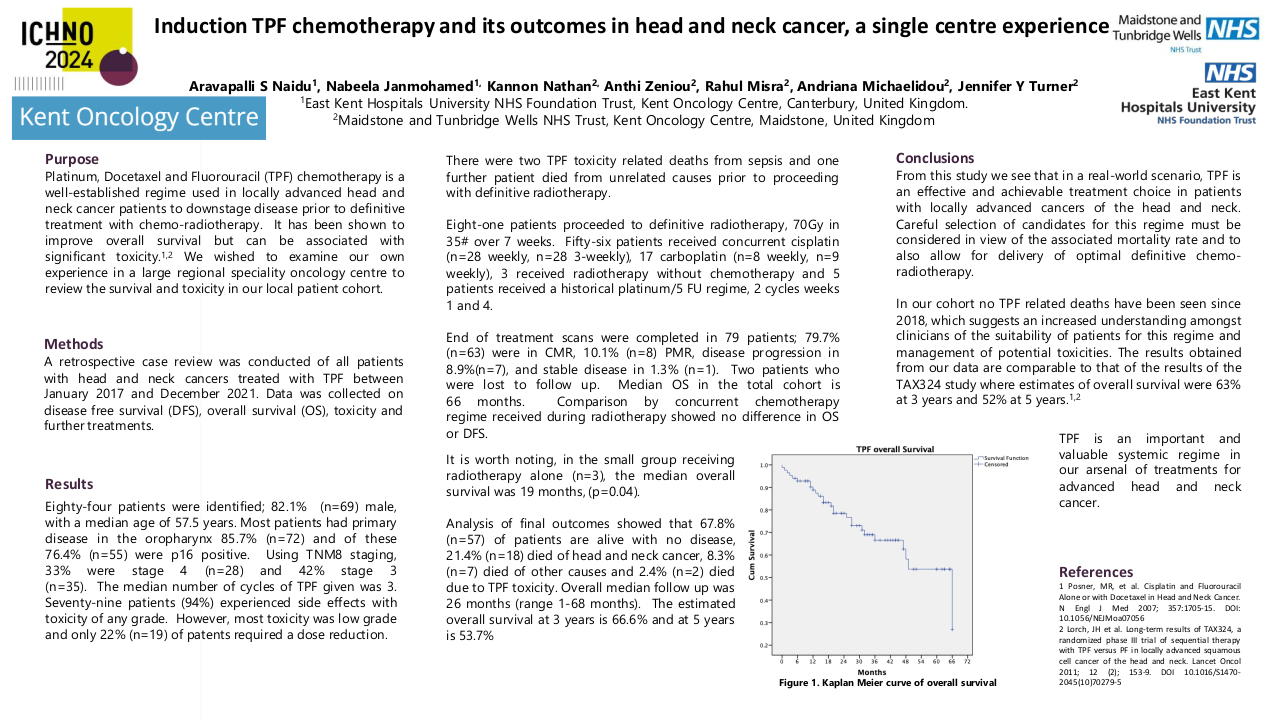
Median overall survival in the total cohort of patients is 66 months. Comparison by the concurrent chemotherapy regime received during radiotherapy showed no difference in overall or disease free survival. However it is worth noting, although the group receiving radiotherapy alone was small, the median overall survival was 19 months, (p=0.04).
Analysis of final outcomes showed that 67.8% (n=57) of patients are alive with no disease, 21.4% (n=18) died of head and neck cancer, 8.3% (n=7) died of other causes and 2.4% (n=2) died due to TPF toxicity. Overall median follow up was 26 months (range 1-68 months). The estimated overall survival at 3 years is 66.6% and at 5 years is 53.7%.
Conclusion
From this study we see that in a real-world scenario, TPF is an effective and achievable treatment choice in patients with locally advanced cancers of the head and neck. However careful selection of candidates for this regime must be considered in view of the associated mortality rate and to also allow for delivery of optimal definitive chemo-radiotherapy. In our cohort no TPF related deaths have been seen since 2018, which suggests an increased understanding amongst clinicians of suitability of patients for this regime and management of potential toxicities. The results obtained from our data are comparable to that of the results of the TAX324 study where estimates of overall survival were 63% at 3 years and 52% at 5 years.1,2 Therefore TPF is an important and valuable systemic regime in our arsenal of treatments for advanced head and neck cancer.
1) Posner, MR et al. Cisplatin and Fluorouracil Alone or with Docetaxel in Head and Neck Cancer. N Engl J Med 2007; 357:1705-15. DOI: 10.1056/NEJMoa070562) Lorch, JH et al. Long term results of TAX324, a randomized phase III trial of sequential therapy with TPF versus PF in locally advanced squamous cell cancer of the head and neck. Lancet Oncol 2011; 12 (2); 153-9. DOI 10.1016/S1470-2045(10)70279-5