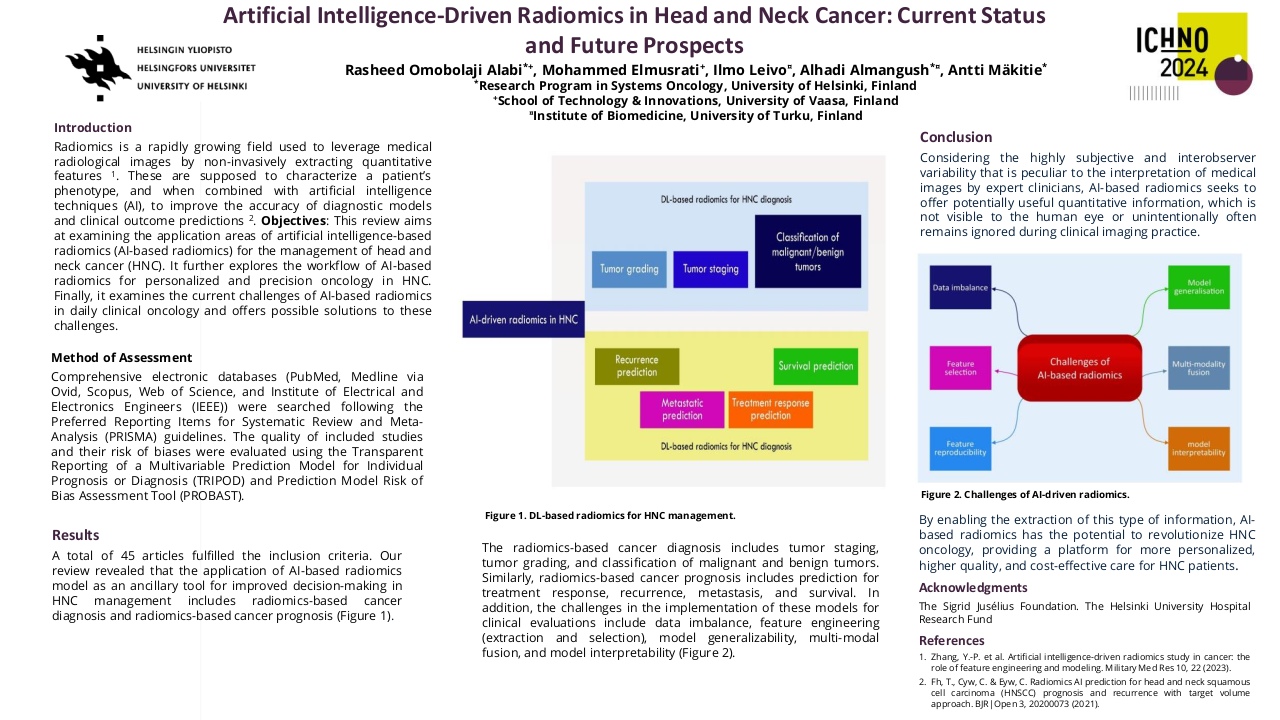
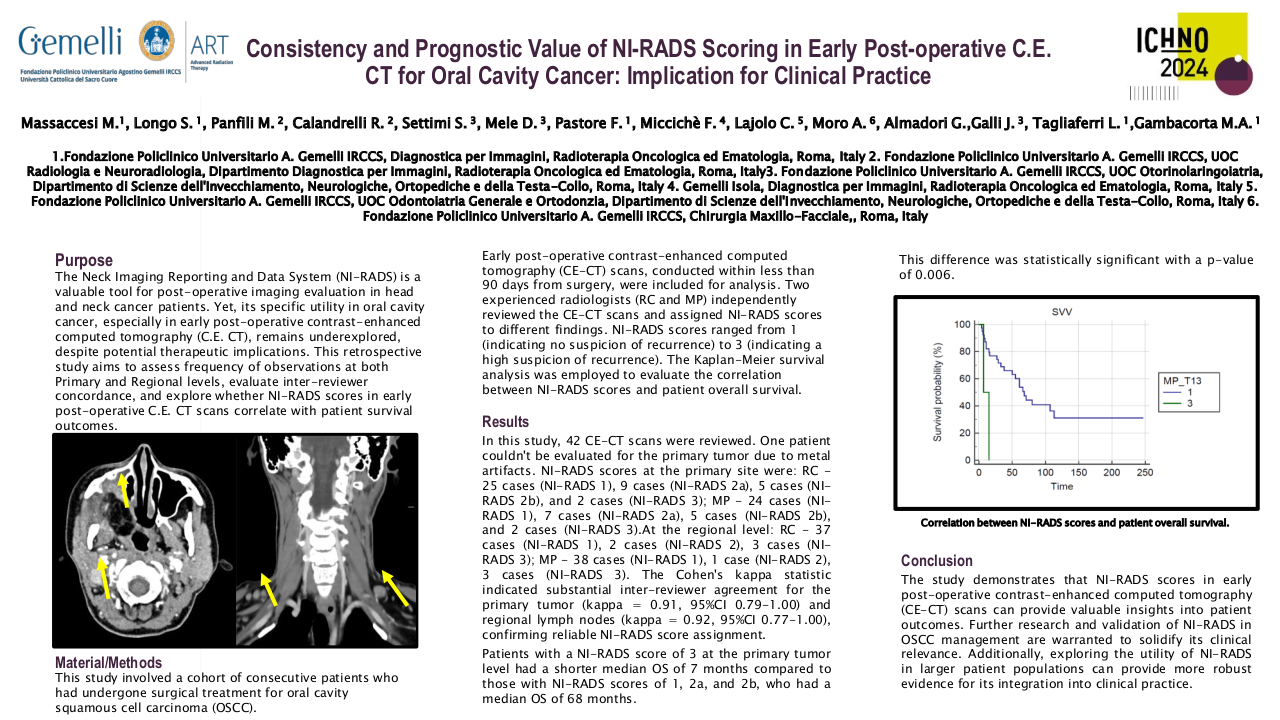
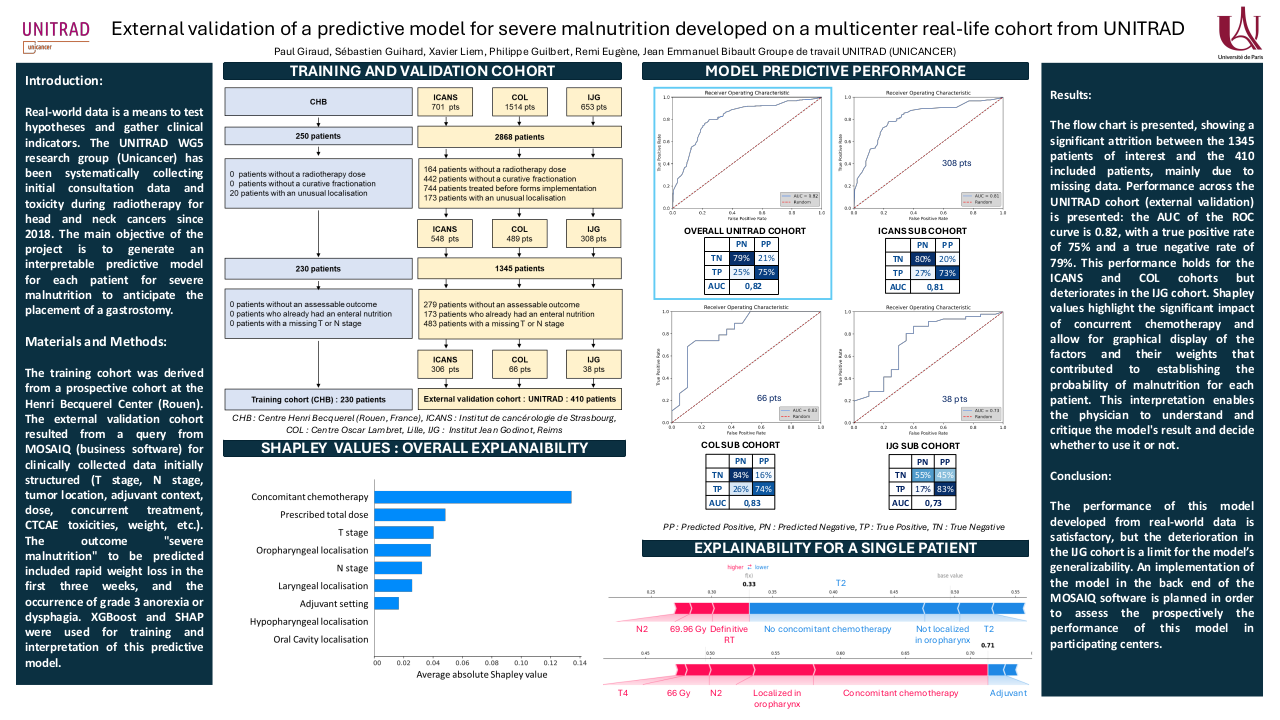
ex-vivo 7T MRI to determine resection margins for tongue cancer resection specimens
Purpose/Objective
The preferred treatment for oral squamous cell carcinoma is complete surgical tumour resection to achieve local-regional control. [1] Complete surgical removal requires an adequate resection margin of at least 5 mm of healthy tissue around the tumour. Unfortunately, international literature reports 30% to 85% inadequate resection margins (i.e., positive and close margins). [2] This study aims to investigate whether adequate tongue cancer resection margins can accurately be determined using ex-vivo high-field magnetic resonance imaging (MRI). This proposed technique could help improve the localisation of inadequate margins during surgery.
Material/Methods
Nine fresh resection specimens of tongue cancer patients were scanned with a small-bore 7 Tesla (7T) MRI scanner (Biospec 70/20, Bruker, Ettlingen, Germany) interfaced with a Philips console. For each resection specimen, four MRI scans were obtained: a 3D T2-weighted (T2W) Turbo Spin Echo (TSE) scan with an isotropic voxel size of 0.3 mm3 and three orthogonal multi-slice T2W TSE scans with an in-plane resolution of 0.125 mm2 and a slice thickness of 1 mm. After completion of the MR protocol, the resection specimens were processed according to routine clinical histopathological workup. The tumour was delineated by a histopathologist (HP) on the hematoxylin and eosin (HE) stained histopathological slices and by two independent radiologists (R1 and R2) on the coronal multi-slice MR scan.
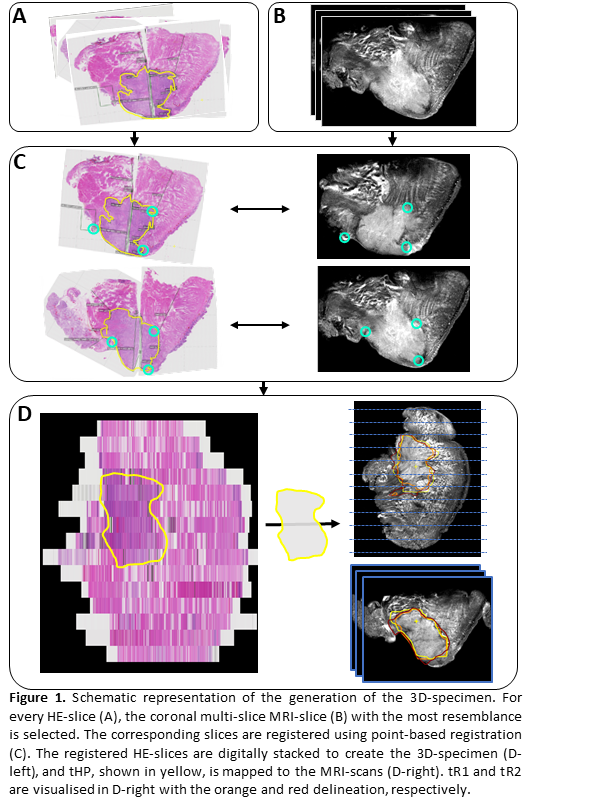
For each patient, a 3D representation of the resection specimen was created using the following three steps: 1) For each HE-slice, the MR-slice with the highest resemblance was found by visual inspection (Fig. 1A, 1B). 2) These corresponding slices were registered using point-based registration based on visual corresponding anatomical points (e.g., specimen contour, tumour-protrusions, mucosa) (Fig. 1C). 3) The registered HE-slices were digitally stacked to create a 3D-specimen of all HE-slices (Fig. 1D). This method to create the 3D-specimen was previously discussed and used in the work by Caldas-Magalhaes et al. [3] The delineated tumour created by the radiologists (tR1 and tR2) and histopathologist (tHP) were interpolated and mapped to the other MRI-scans (i.e., sagittal, axial, and 3D TSE scan) (Fig. 1D).
The accuracy of the inadequate margin detection by R1 and R2 was determined using two different methods. First, the minimal resection margins in all five directions (anterior, posterior, craniomedial, caudolateral and central) were measured based on tR1 and tR2 and compared to the measurements reported by the HP. Second, the 95th percentile Hausdorff distance (HD95) of the overestimation (O-HD95) and underestimation (U-HD95) of tR1 and tR2 was calculated with respect to the ground truth (tHP) in all five directions. The relationship between the measured resection margins based on tR1, tR2 and tHP was evaluated using Pearson’s correlation coefficient (PCC). Only the craniomedial, caudolateral, and central resection margins were included in the PCC analysis.
Results
According to the histological report, one of the nine resection specimens was adequately resected. Based on the in-plane measurements, R1 and R2 were able to accurately determine which tumours were (in)adequately removed in 7/9 and 8/9 cases. Two of these cases were inadequate but considered adequate by the radiologists, and the other case was determined inadequate by R1 but adequate according to the HP. All these three incorrect cases had a T-stage of 1.
Based on the tumour segmentation, the sensitivity and specificity of the detection of inadequate margins with respect to the final histopathological report were 77% and 50% for R1 and 65% and 57% for R2, respectively. The median U-HD95 and O-HD95 for R1 were 0.9 mm (range: 0.0-1.7 mm) and 2.5 mm (range: 0.6-11.8 mm). For R2, the median U-HD95 and O-HD95 were 0.5 mm (range: 0.3-5.3 mm) and 2.5 mm (range: 1.2-6.6 mm).
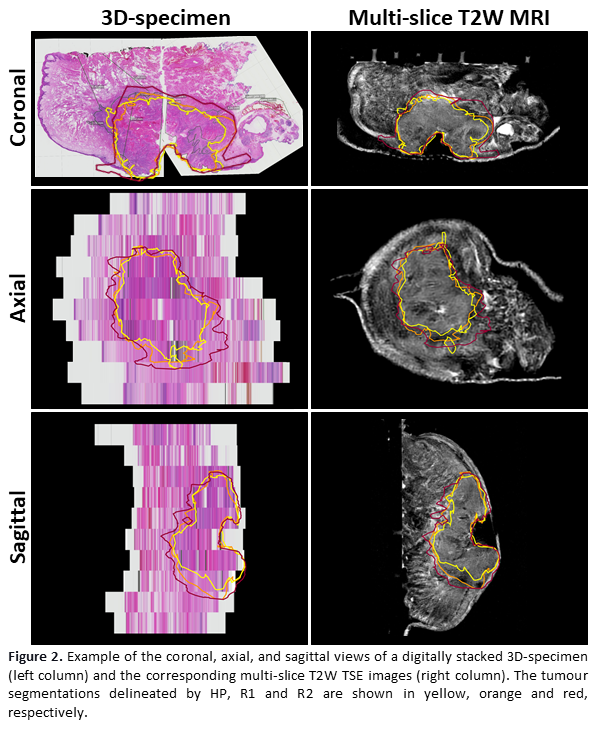
The PCC of the measured margins between the HP and two radiologists was 0.67 and 0.71 for R1 and R2, respectively. Between both radiologists, the PCC of the measured margins was 0.75. Figure 2 shows an example of a 3D-specimen and the corresponding MRI-slices.
Conclusion
Ex-vivo 7T MRI enables accurate margin predictions for tongue cancer resection specimens. The radiologists’ assessment of in- and adequate resections based on ex-vivo 7T MRI generally aligns with the histopathology reports. The sensitivity of both radiologists for adequate margin detection is reasonable. However, the specificity is low, which might be caused by the radiologists’ overestimation of the tumour on ex-vivo 7T MRI. This study offers a proof of principle for future studies to validate and further improve the detection of inadequate margins for oral cavity resection specimens using ex-vivo 7T MRI. This technique could provide guidance for surgeons to localise inadequate margins and enable them to perform accurate re-resections during surgery.
1. Chinn SB, Myers JN. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J Clin Oncol. 2015 Oct 10;33(29):3269-76.2. Smits RWH, Koljenovic S, Hardillo JA, Ten Hove I, Meeuwis CA, Sewnaik A, et al. Resection margins in oral cancer surgery: room for improvement. Head Neck 2016;38:E2197–203.3. Caldas-Magalhaes J, Kasperts N, Kooij N, van den Berg CA, Terhaard CH, Raaijmakers CP, Philippens ME. Validation of imaging with pathology in laryngeal cancer: accuracy of the registration methodology. Int J Radiat Oncol Biol Phys. 2012 Feb 1;82(2):e289-98.