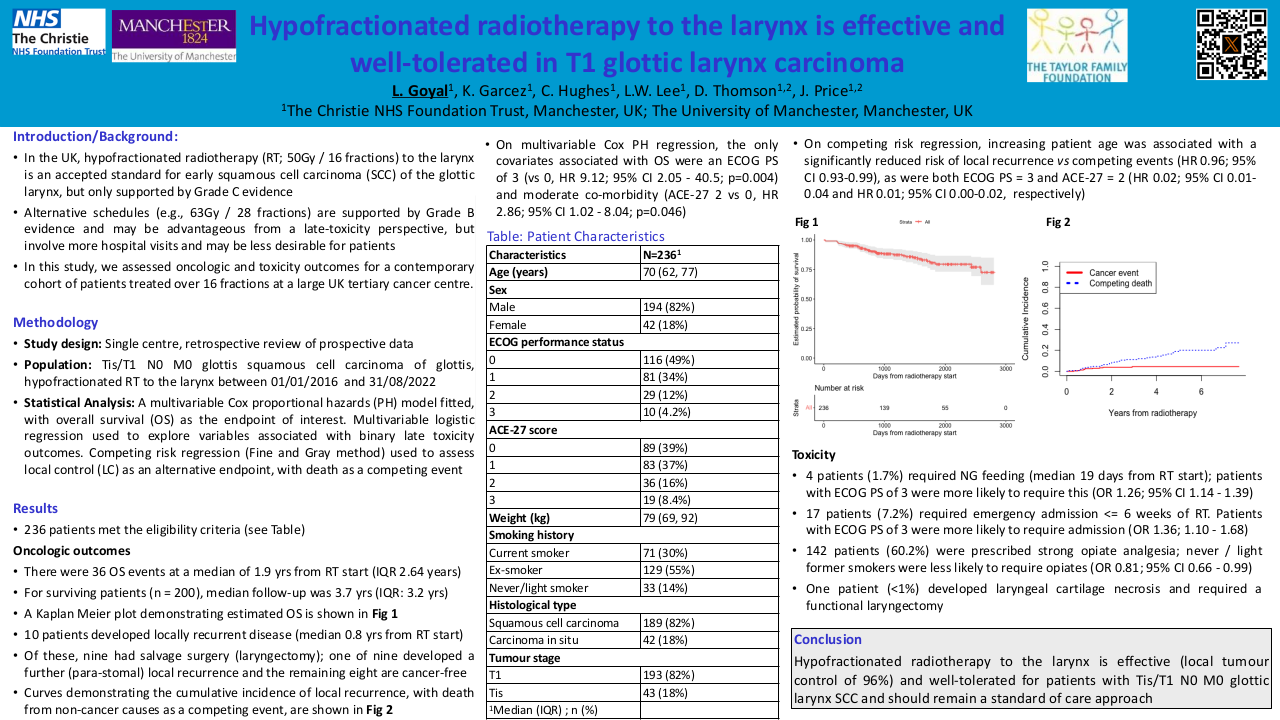
Patient-oriented, individualized follow-up in head and neck cancer (DeIntensiF randomized trial NCT05388136)
Purpose/Objective
Approximately 70% of head and neck cancer (HNC) patients present with locoregionally advanced disease. The curative rate for early disease is 80-95%; for advanced tumors, locoregional recurrence rate remains at about 50-60% despite advances in treatment, and 20-30% will have distant metastases. Further, patients will develop a second primary malignancy (SPM) with a rate of 2-4% per year.
Follow-up (FU) is important to detect recurrence (REC) and SPM at an early stage, to enable effective salvagetherapy, manage treatment-related sequelae, and provide functional rehabilitation and psychosocial support.
In the absence of high-level evidence, there is no clear international consensus in FU regimens. There are only retrospective studies addressing this topic, mostly showing no difference in overall survival between patients with REC detected during routine FU and symptom-driven self-referral visits. The value of imaging is also subject of debate. Moreover, many of the hitherto published studies did not include the logistical, psychological and financial consequences and the relevant cost evaluations in today’s healthcare systems facing increasing financial pressure.
We propose a large multicenter, randomized prospective trial in HNC patients with complete remission 6 months after curative treatment to compare two FU schemes differing in frequency of scheduled clinical examinations and imaging. We hypothesize that implementing an individualized de-intensified FU with active patient involvement does not differ from a conventional regular FU in terms of death from any cause up to 5 years (=primary endpoint). We also hypothesize that symptom-driven self-referral FU visits have a higher diagnostic yield in detection of REC/SPM than regular scheduled clinical and radiological examinations. Consequently, we assume that fewer scheduled exams in well-instructed patients will not lead to worse outcome. The secondary objectives are the comparison of death from HNC and any cancer, detection of first REC/SPM, health-related quality of life, fear of recurrence, compliance with FU assessments, number of visits and HNC-specific health-care utilization.
The objective of the herein presented Pilot 1 study was to assess the feasibility of patients’ recruitment, motivation for trial participation and compliance in completing a monthly, paper-based symptoms’ monitoring (patient-reported outcome [PRO], symptom tracker). This Pilot is supported by Swiss Cancer Research.
Material/Methods
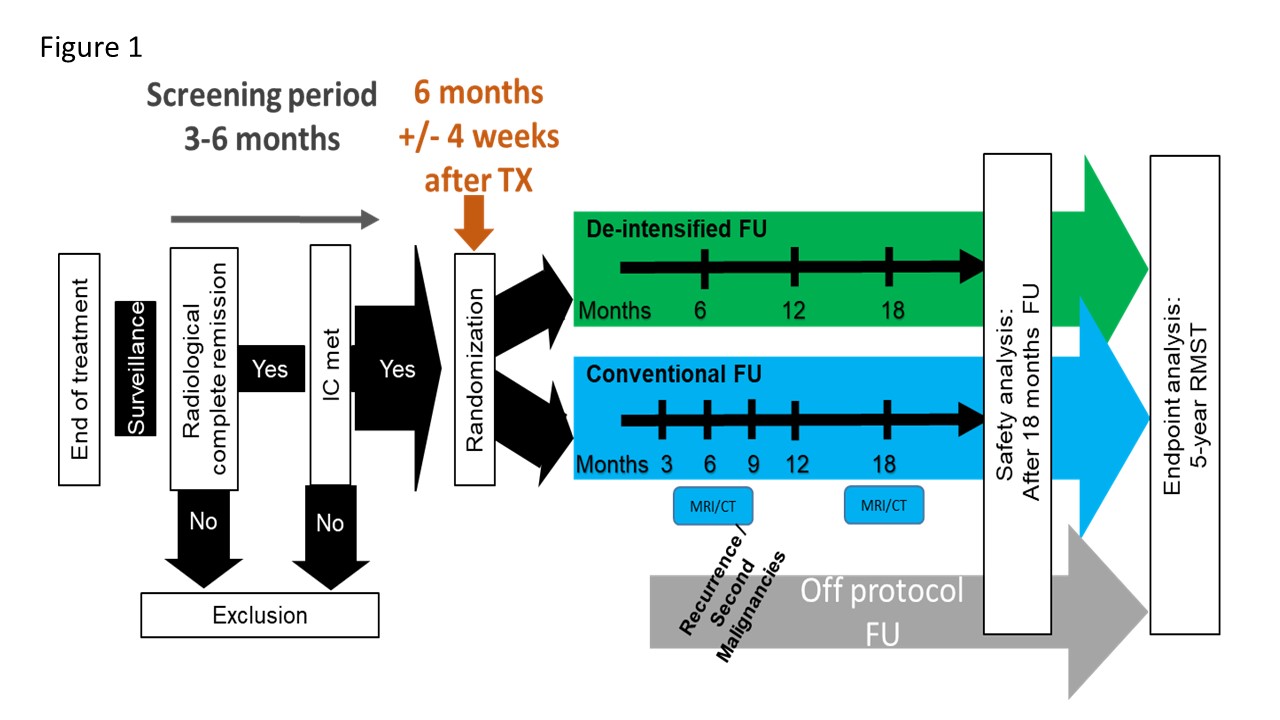
The study design is shown in Figure 1 (RMST: restricted mean survival time).
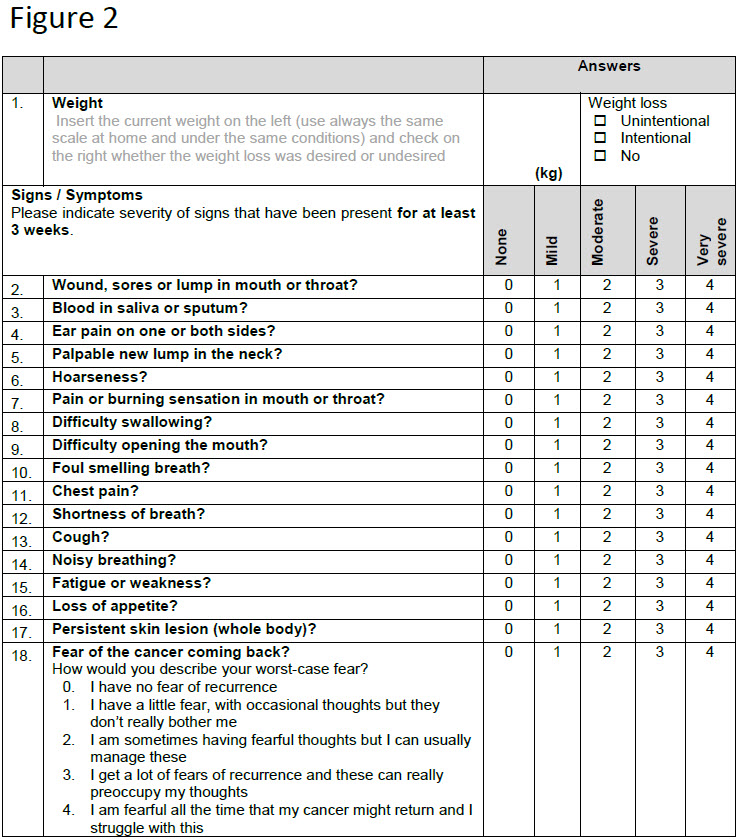
The main study is a randomized-controlled combined non-inferiority and superiority trial with explicit Pilot 1 and 2. After curative treatment, participants are randomized to an individualized de-intensified FU with monthly symptoms’ monitoring (Figure 2) (experimental arm) or to standard FU.
Alerting symptoms possibly indicating REC/SPM or non-completion of the PROs will result in an urgent clinical FU appointment in the experimental arm. Minimal FU within Pilot 1 is 12 months (as opposed to 60 months in the main trial). Recruitment was done in three Swiss tertiary referral centers, which committed to enroll at least 20 patients during one year.
Results
The primary aim of Pilot 1, evaluating the feasibility of patient recruitment, has been confirmed faster than expected (20 committed patients randomized after 7 and 29 patients randomized after 11 months accrual time, respectively). Six unscheduled visits were triggered by our paper-based PRO. Within Pilot 1, a prescreening survey was conducted to better understand the specific motivation of patients to participate in the trial or not. We surveyed 41 potential participants of which 27 (66%) agreed to participate. The potential reduction in imaging was the main reason for the patients to participate in the trial (52%). Additionally, we collected feedback on the design of the paper-based PRO questionnaire at the 6-month FU visit when participants had already gained some experience. Participants expressed that the PRO questionnaire was easy to understand and comprehensive, thus facilitating them to communicate with their corresponding study center. The completion time for the PRO was between 5-10 minutes in 63% of the participants. In addition, 63% of the patients were in favor of transitioning the paper-based PRO to an electronic version (ePRO), the other 37% felt unsure about using an ePRO. Interim compliance data will be presented.
Conclusion
The recruitment and symptoms’ monitoring for HNC patients have been proofed as feasible. Pilot 2 is in planning to allow for a smooth continuation of Pilot 1 with following specific goals: 1) to expand the trial to 12 Swiss and 4 European sites; 2) to recruit up to 200 participants; 3) to implement a web-based version of the symptom tracker (ePRO); 4) to develop an enhanced training strategy for HNC patients; 5) to evaluate the usability of the ePRO; and 6) to assess the safety of omitting systematic lung imaging.
Nguyen NA, Ringash J. Head and Neck Cancer Survivorship Care: A Review of the Current Guidelines and Remaining Unmet Needs. Curr Treat Options Oncol. 2018;19:44.