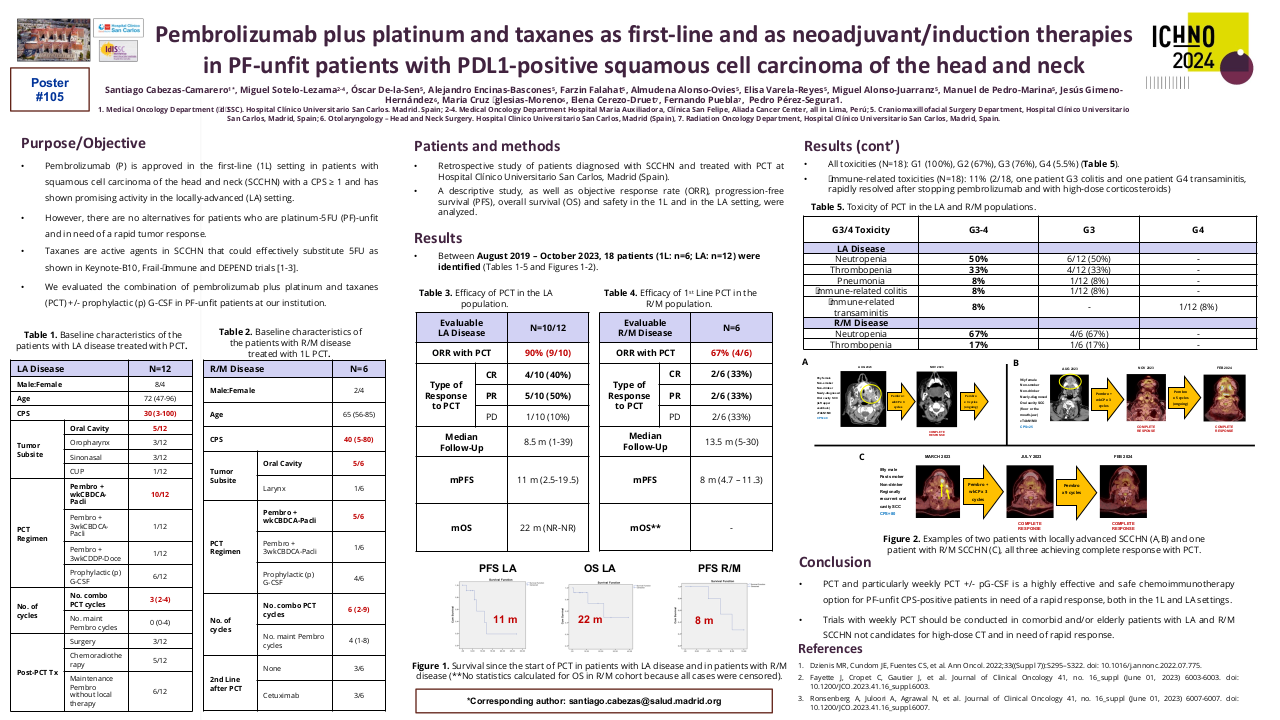
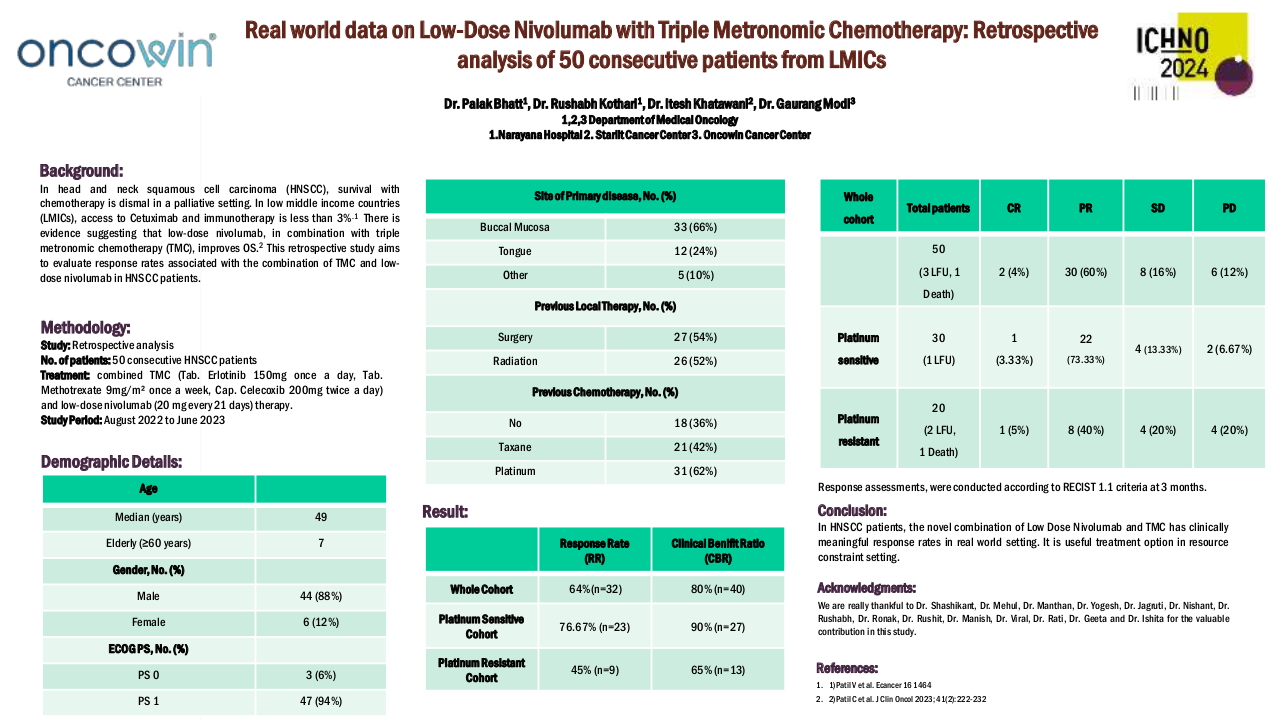
Influence of chemotherapy regimens prior to nivolumab on the survival of recurrent/metastatic SCCHN.
Purpose/Objective
Since the incorporation of immunotherapy in the treatment of recurrent/metastatic SCCHN, the best sequencing with existing chemotherapy regimens has been investigated. In the KESTREL trial, patients treated at progression with immunotherapy had a 10-month increase in OS. The same is observed in the TTCC-2019-02 study, real world evidence taxol/cetuximab of TTCC group, in which the increase in survival was 13 months in patients treated with immunotherapy at progression. But, in the TPEX trial, patients treated in the experimental arm with immunotherapy at progression showed a 7-month overall survival superior to those treated with chemotherapy at progression, while those treated with EXTREME and at progression with immunotherapy the increase in OS was only of 2 months.
Patients with recurrent/metastatic tumors usually undergo multiple treatments. This study aims to determine the relationship between overall survival (OS), survival since treatment with nivolumab (Snivo) and progression-free survival (PFS) depending on the chemotherapy drug received in previous lines (taxanes and/or cetuximab).
Material/Methods
This is a retrospective, observational study that collects patient characteristics, tumor and previous treatments of all patients treated with nivolumab in care at the Sant Joan de Reus University Hospital from January 2018 to April 2023. Information has been collected from the digitized medical history.
The relationship between previous treatments and survival (overall and progression-free) has been determined using the Kaplan Meier method and the log-rank test to compare the survival rate between groups. A p-value <0.05 has been considered statistically significant. To determine the possible variables influencing survival, the univariate and multivariate Cox proportional hazard model was used. The response rate to nivolumab and according to previous treatments has been calculated.
Results
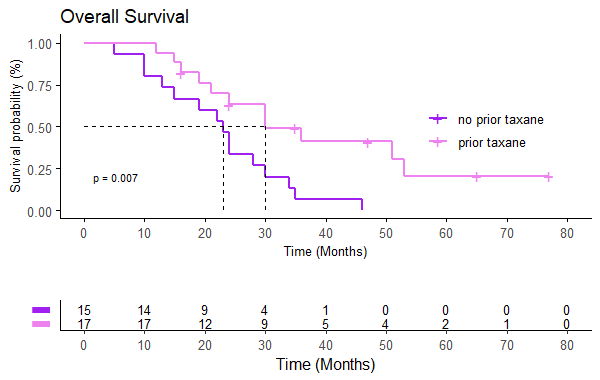
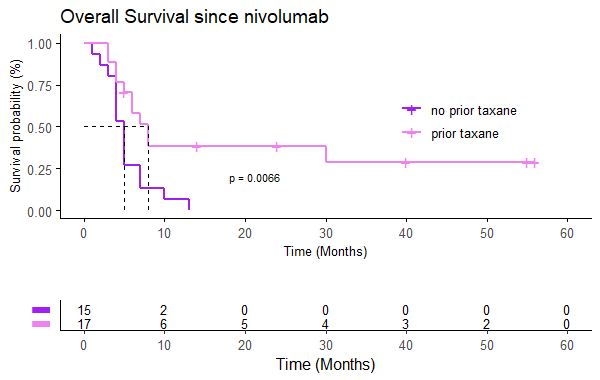
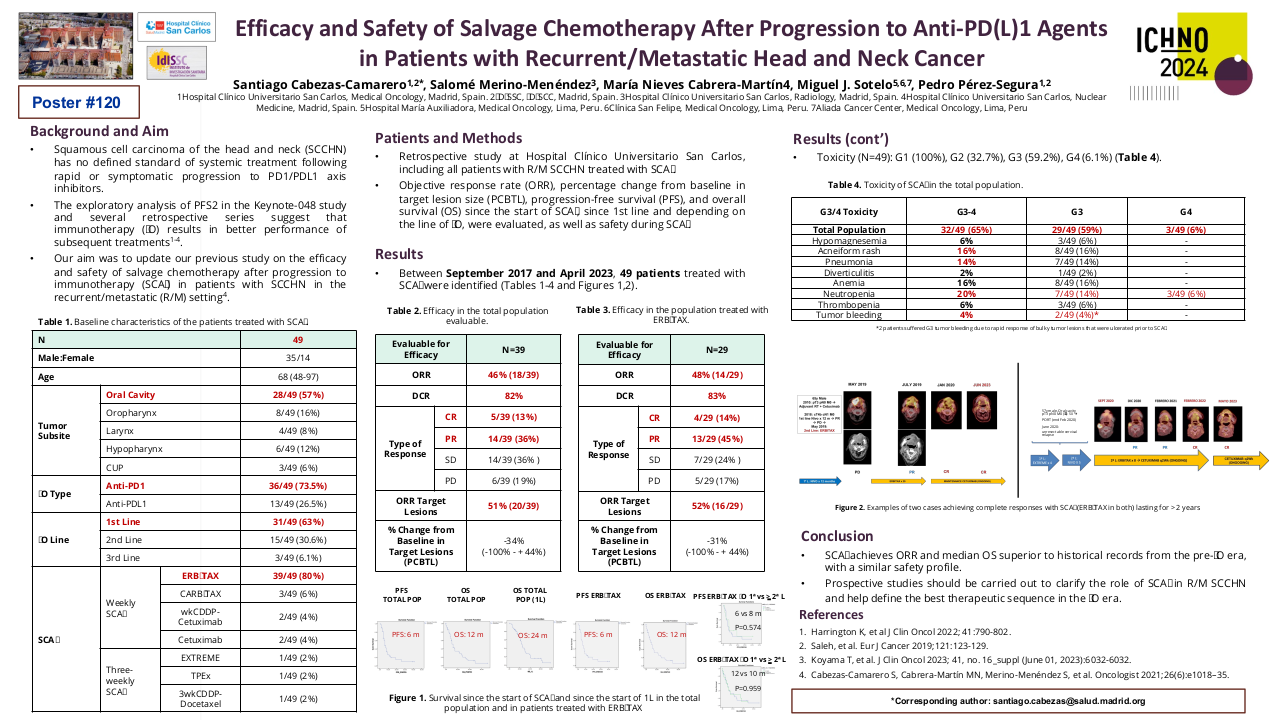
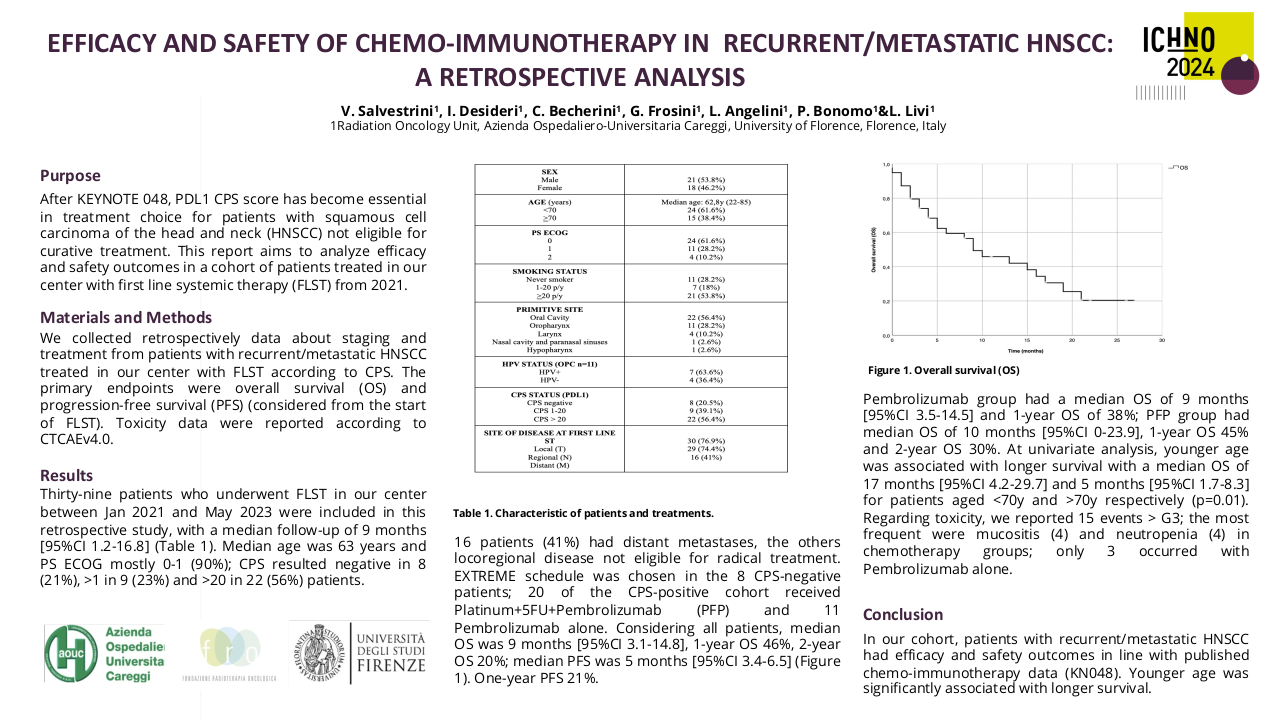
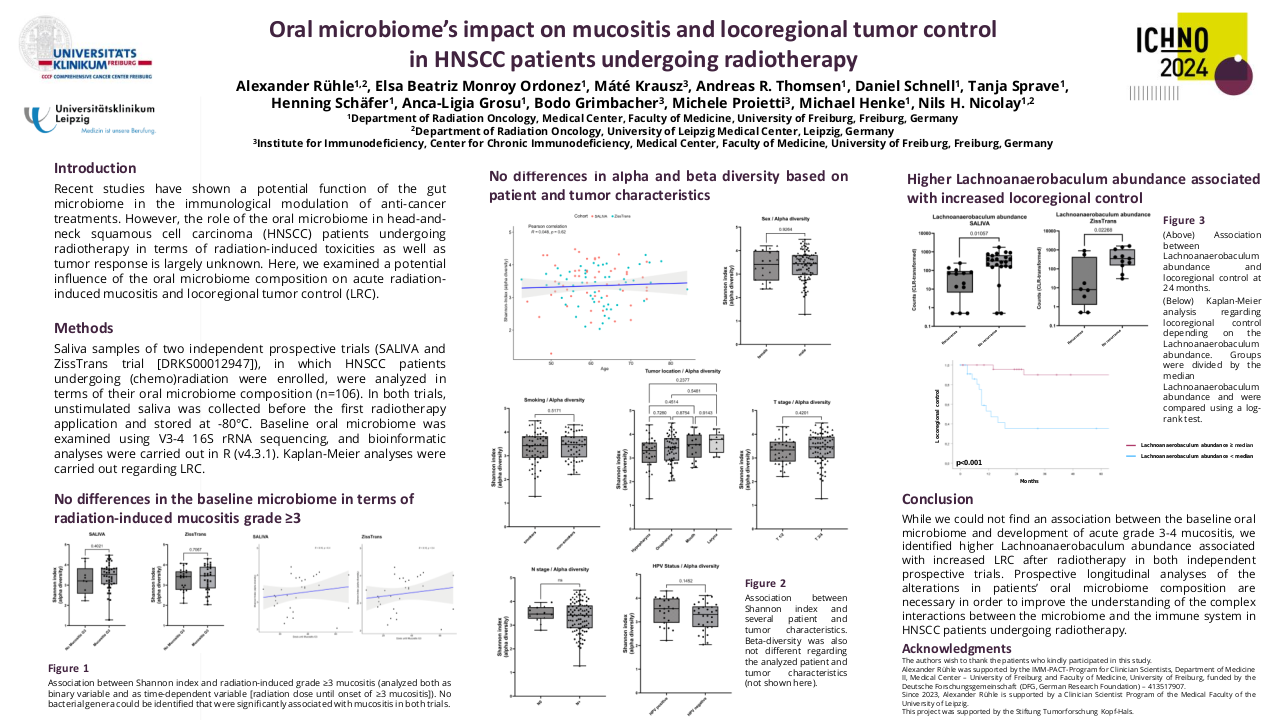
32 patients have been included, 90% men with an average age of 51 years and the majority with significant toxic habits. 46.9% are located in the oral cavity, 15.6% stage IVC at diagnosis and 25% p16 positive. CPS could only be determined in 6 cases. 37.5% present local progression and 40.6% local and distant progression. 53.1% of patients have undergone regimens with taxanes and 59.4% with cetuximab. 10 patients have been treated with nivolumab in the first line of recurrent/metastatic disease.
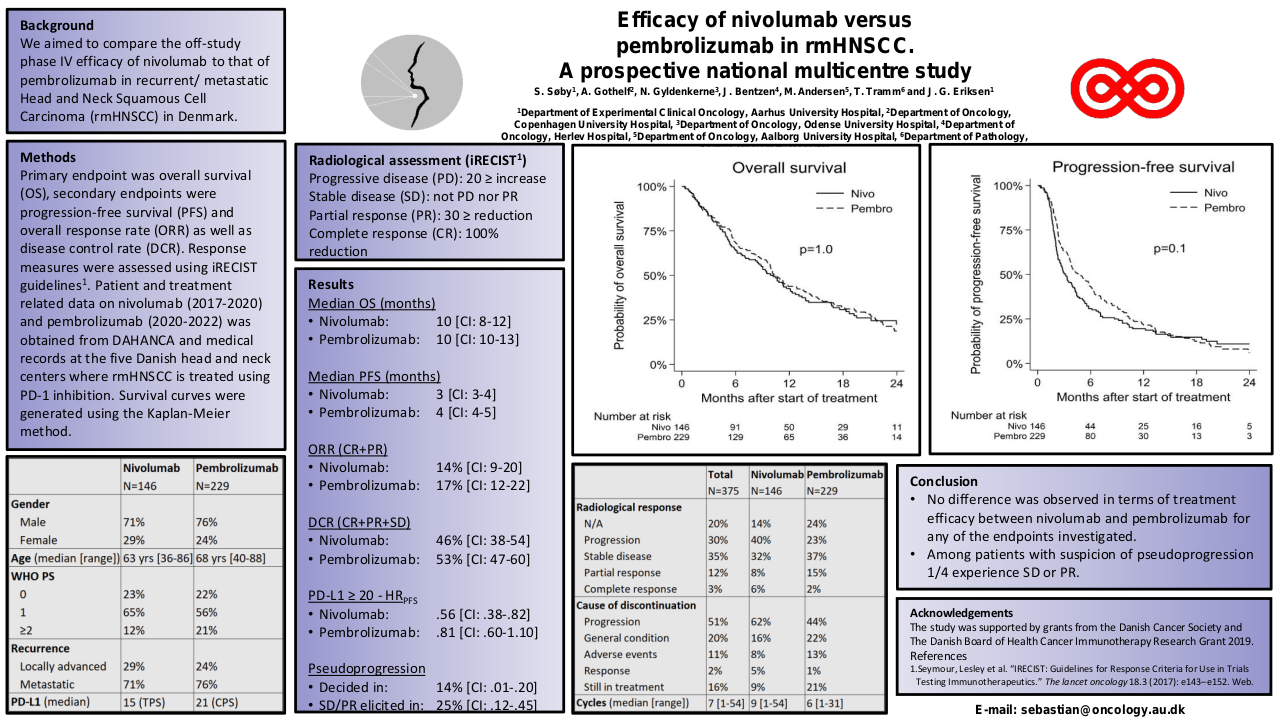
The median OS is 24 months (95% CI 22-35), mSnivo is 5.5 months (95% CI 5-8) and mPFS is 4 months (95% CI 3-5). There are no differences depending on whether nivolumab is administered in the first line or successive (p 0.17) but if previously treated with taxanes the OS is superior compared to those who are not (30 vs 23 months, p=0.007, HR 0.33). The same is observed in Snivo (8 vs 5 m, p=0.006, HR 0.34). There are no differences in PFS with nivolumab if it has been previously treated with taxanes (p=0.15). There is also no difference in OS, Snivo and PFS if previously treated with cetuximab (p=0.98, p=0.097 and p=0.15 respectively) but there is a trend towards higher Snivo in patients not previously treated with cetuximab.
Disease control rate of 34.4% has been observed, with 4 complete responses (12.5%) and ORR 21.9%. There are no statistically significant differences according to previous treatment, but all CRs are in the previous taxane arm and there is a trend towards greater CR, ORR and DCR in those not previously treated with cetuximab.
In the univariate Cox hazard model, only prior treatment with taxanes has been identified as an independent survival factor, so a multivariate analysis has not been carried out.
Conclusion
Treatment with taxanes prior to nivolumab has been identified as an independent survival factor, presenting an improvement in both OS and survival since nivolumab with a reduction in the risk of death of more than 65%. The results are consistent with those observed in the CheckMate 141, TPEx studies or real world evidence taxol/cetuximab study of TTCC group. Since this is an observational study that suggests a sequencing scheme, these data would have to be validated in future clinical trials.
Psyrri A. Fayette J, Harrington K. Durvalumab with or without tremelimumab versus the EXTREME regimen as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck: KESTREL, a randomized, open-label, phase III study. Ann Oncol. 2023 Mar;34(3):262-274Cirauqui Cirauqui B, Martinez Trufero J, Plana Serrahima M, et al. Real Wolrd Evidence of First-line Cetuximab (CX) plus Paclitaxel (PX) in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (SCCHN) - TTCC-2019-02. Annals of Oncology (2022) 33 (suppl_7): S295-S322.Guigay J, Aupérin A, Fayette J, et al. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014-01 TPExtreme): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol 2021;22:463-475.Ferris R. Blumenschein G, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375:1856-1867