Pembrolizumab plus platinum and taxanes as first-line and as neoadjuvant/induction therapies in PF-unfit patients with PDL1-positive squamous cell carcinoma of the head and neck
Purpose/Objective
Pembrolizumab (P) is approved in the first-line (1L) setting in patients with squamous cell carcinoma of the head and neck (SCCHN) with a CPS ≥ 1 and has shown promising activity in the locally-advanced (LA) setting. However, there are no alternatives for patients who are platinum-5FU (PF)-unfit and in need of a rapid tumor response. Taxanes are active agents in SCCHN that could effectively substitute 5FU as shown in Keynote-B10, Frail-Immune and DEPEND trials [1-3]. We evaluated the combination of pembrolizumab plus platinum and taxanes (PCT) +/- prophylactic (p) G-CSF in PF-unfit patients at our institution.
Material/Methods
Retrospective study of patients diagnosed with SCCHN and treated with PCT at Hospital Clínico Universitario San Carlos, Madrid (Spain). A descriptive study, as well as objective response rate (ORR), progression-free survival (PFS), overall survival (OS) and safety in the 1L and in the LA setting, were analyzed.
Results
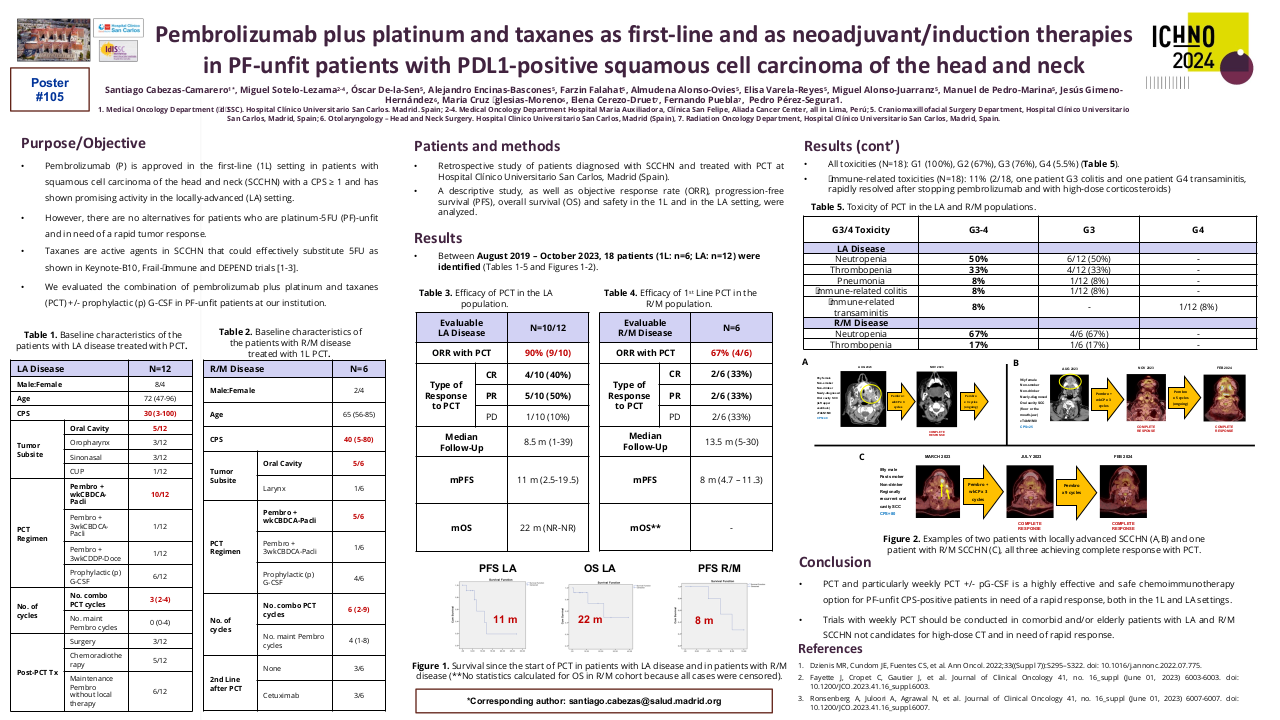
Within the period August 2019 – October 2023, 18 patients (1L: n=6; LA: n=12) were identified. Among 1L: Male/Female: 2/4; age: 65y (56-86). Subsite: oral cavity (OC): n=5, larynx (n=1). Median CPS: 40 (5-80). No. combo PCT cycles: 6 (2-9). No. maintenance P cycles: 4 (1-8). ORR: 67% (2 CR, 2 PR, 2 SD). After a median follow-up (F-U) of 9 months (m) (1-25), median PFS and OS were 8 m (4.7 - 11.3) and NR (NR-NR), respectively. pG-CSF in 4/6 pts. G3/4 toxicity: G3 neutropenia: 4/6, G3 thrombopenia: 1/6. There were no toxic deaths. Among LA: Male/Female: 8/4; age: 72y (47-96). P + wkCBDCA-paclitaxel: n=10/12; P + 3wkCBDCA-paclitaxel: n=1/12, P + 3wkCDDP-docetaxel: n=1/12. Subsite: oropharynx: n=3, OC: n=5, CUP: n=1, Sinonasal: n=3. Median CPS: 30 (3-100). No. combo PCT cycles: 3 (2-4). No. maintenance P cycles: 0 (0-4). ORR after PCT (n=10): 90% (4 CR, 5 PR, 1 PD). Post-PCT Tx: Surgery: n=3; CRTx: n=5; Maint P: n=6. After a median follow-up (F-U) of 4 m (0-34), median PFS and OS were 6 m (NR - NR) and NR (NR-NR), respectively. pG-CSF in 6/12 pts. G3/4 toxicity: G3 neutropenia: 6/12, G3 thrombopenia: 4/12, G3 pneumonia: 1/12, G3 IR-colitis: 1/12, G4 IR-transaminitis: 1/12. There were no toxic deaths.
Updated results for the LA population (as of March 2024) will be presented at the meeting.
Conclusion
PCT and particularly weekly PCT +/- pG-CSF is a highly effective and safe chemoimmunotherapy option for PF-unfit patients in need of a rapid response and a positive CPS, both in the 1L and LA settings. Trials with weekly PCT should be conducted in comorbid and/or elderly patients with LA and R/M SCCHN not candidates for high-dose CT and in need of rapid response.
1. Dzienis MR, Cundom JE, Fuentes CS, et al. 651O-Pembrolizumab (pembro) + carboplatin (carbo) + paclitaxel (pacli) as first-line (1L) therapy in recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): Phase VI KEYNOTE-B10 study. Ann Oncol. 2022;33((Suppl 7)):S295–S322. doi: 10.1016/j.annonc.2022.07.775.2. Fayette J, Cropet C, Gautier J, et al. Results of the multicenter phase II FRAIL-IMMUNE trial evaluating the efficacy and safety of durvalumab combined with weekly paclitaxel carboplatin in first-line in patients (pts) with recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) not eligible for cisplatin-based therapies. Journal of Clinical Oncology 41, no. 16_suppl (June 01, 2023) 6003-6003. doi: 10.1200/JCO.2023.41.16_suppl.6003.3. Ronsenberg A, Juloori A, Agrawal N, et al. Neoadjuvant nivolumab, paclitaxel, and carboplatin followed by response-stratified chemoradiation in locoregionally advanced HPV negative head and neck squamous cell carcinoma (HNSCC): The DEPEND trial. ournal of Clinical Oncology 41, no. 16_suppl (June 01, 2023) 6007-6007. doi: 10.1200/JCO.2023.41.16_suppl.6007.