Efficacy of nivolumab versus pembrolizumab in rmHNSCC. A prospective national multicenter study.
Purpose/Objective
The PD-1 inhibitor, nivolumab, was introduced to Danish PD-L1 positive patients as second-line treatment for recurrent/metastatic head and neck squamous cell carcinoma (rmHNSCC) in 2017. In 2020 pembrolizumab was introduced as first-line treatment for PD-L1 positive disease. Aim of the present study was to determine the real-life efficacy of single drug treatment with pembrolizumab compared to nivolumab.
Material/Methods
Patients were eligible if they were treated between 2017 and end of 2022, had histologically confirmed PD-L1 positive rmHNSCC, and had received either pembrolizumab or nivolumab. Patient, tumor and treatment related data were collected prospectively from patient files at the five Danish head and neck cancer centers and from the DAHANCA database. iRECIST was used for treatment response evaluation, and PD-L1 expression was determined using tumor proportion score (TPS) for nivolumab and combined proportion score (CPS) for pembrolizumab according to international recommendations.
Only patients treated with single agent PD-1 inhibition were eligible for the present study.
Descriptive statistics were used to describe patient, tumor and treatment. Endpoints were response rate (RR), overall survival (OS) and progression-free survival (PFS), calculated from start of treatment to date of event or censoring. Survival was estimated by the Kaplan-Meier method. Analyses were two-sided and p<0.05 were considered significant.
Results
In total 375 patients were identified out of which 229 (61%) received pembrolizumab and 146 (39%) nivolumab. At baseline, the median age was 68 years for pembrolizumab and 63 years for nivolumab (p<0.001). The two groups did not differ significantly in terms of gender with 74% being male and 26% female or in terms of type of recurrence, where metastatic disease was present in 74% of patients while the remaining 26% had locally advanced cancers. The median number of treatment cycles administered was 5 (range: 1-54) for nivolumab and 5 (range: 1-31) for pembrolizumab.
In patients treated with pembrolizumab, 2% [95% CI: 1-6%] experienced complete remission and 19% [95% CI: 14-26%] had partial response. In the nivolumab group 7% [95% CI: 4-13%] achieved complete remission and 9% [95% CI: 5-15%] had partial response.
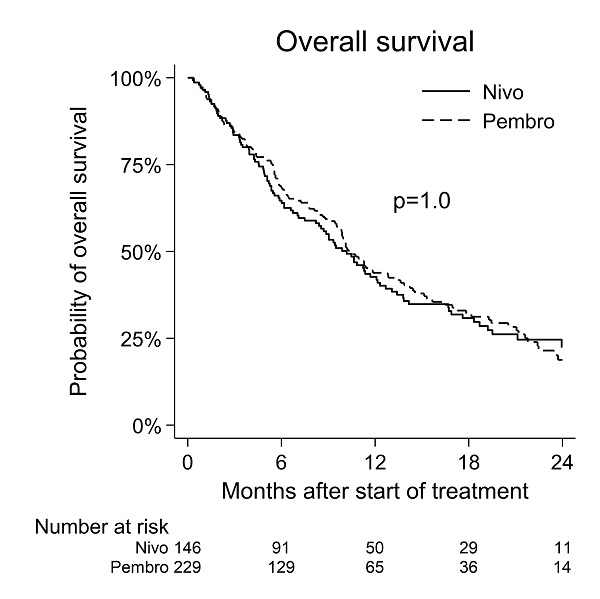
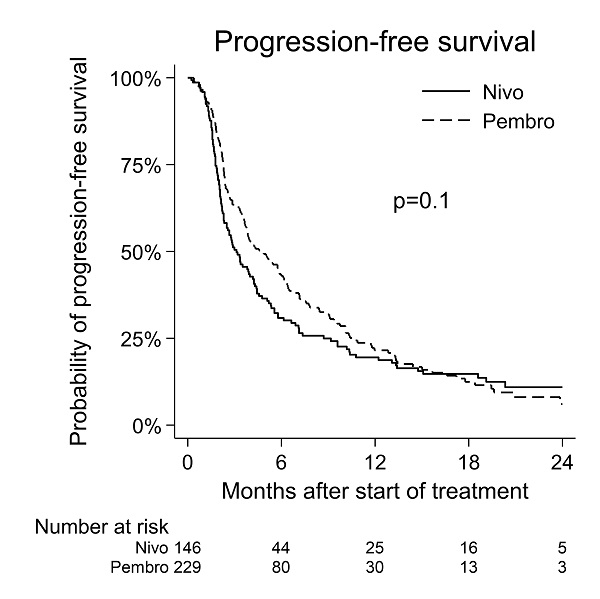
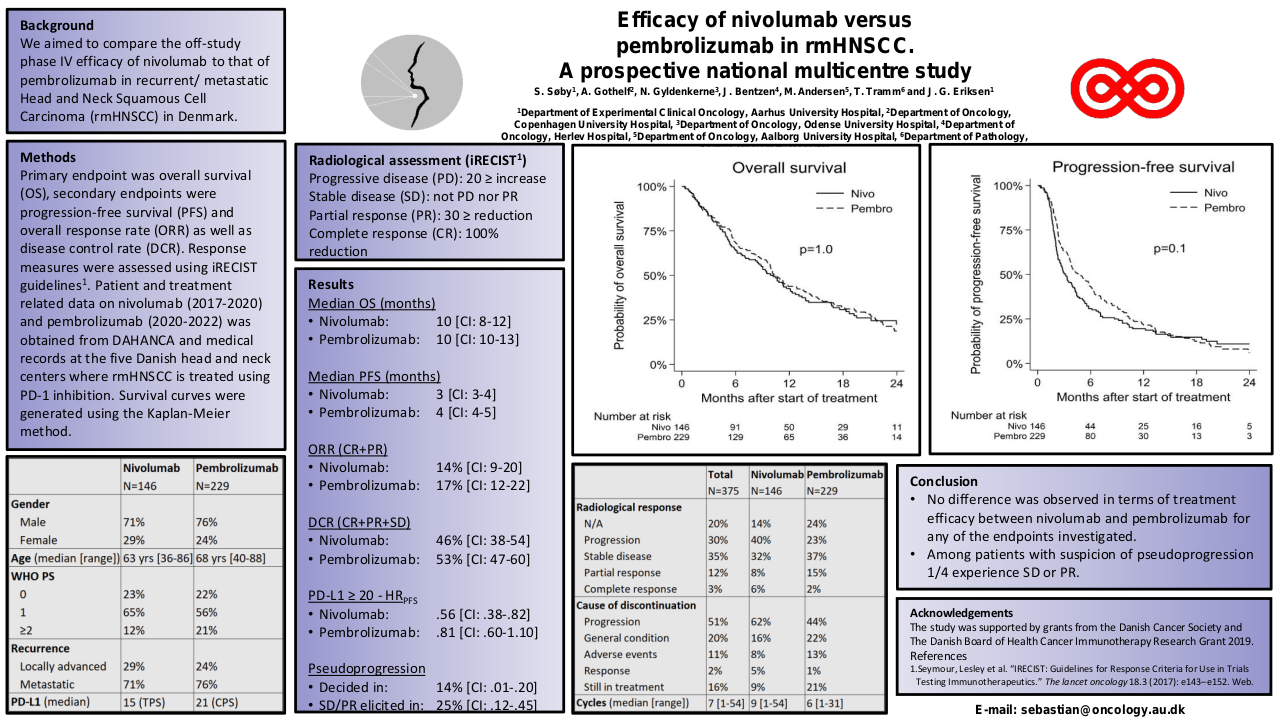
A median OS of 10 months [95% CI: 10-13 Mo.] and a median PFS of 5 months [95% CI: 4-6 Mo.] was observed for pembrolizumab. For nivolumab median OS was 10 months [95% CI: 9-12 Mo.] and median PFS 3 months [95% CI: 3-4 Mo.]. No significant difference in efficacy was observed between the two PD-1 inhibitors using OS or PFS as endpoints (figure 1+2).
WHO performance status (PS) showed an impact on treatment outcome. In the pembrolizumab group, patients with WHO PS=1 had a significant worse outcome (compared to WHO PS=0), HR=1.8 [95% CI: 1.1-3.0] (p=0.01), using OS as endpoint and HR=3.4 [95% CI: 2.0-5.9] (p<0.001) for patients with WHO PS≥2 (compared to WHO PS=0). Using PFS as endpoint, the WHO PS=1 group did worse compared to the WHO PS=0 group, HR=1.6 [95% CI: 1.1-2.4] (p=0.03) while HR=1.7 [95% CI: 1.1-2.7] (p=0.03) was observed for patients with WHO PS≤2 (compared to WHO PS=0).
In the nivolumab group, patients with WHO PS=1 had a significant worse outcome (compared to WHO PS=0), HR=2.9 [95% CI: 1.6-5.2] (p=0.001) using OS as endpoint and HR=10.1 [95% CI: 4.8-21.5] (p<0.001) for WHO PS≤2 (compared to WHO PS=0). For the endpoint PFS patients with WHO PS=1 had a HR=2.3 [95% CI: 1.4-3.7] (p=0.001) while HR=5.3 [95% CI: 2.7-10.4] (p<0.001) for WHO PS≤2 (both endpoints compared to WHO PS=0).
PD-L1 expression did not overall seem to bear any impact on treatment outcome. For patients with TPS ≥ 20% or CPS ≥ 20, HR=0.9 [95% CI: 0.6-1.2] (p=0.4) and HR=0.8 [0.6-1.1] (p=0.2) was obtained for the endpoints OS and PFS respectively. For patients treated with nivolumab using OS as endpoint HR=1.0 [95% CI: 1.0-1.0] (p=0.4) was seen, whereas PD-L1 expression ≥ 20% using PFS as endpoint showed a reduced risk of progression, HR=0.6 [95% CI: 0.4-0.8] (p=0.003).
Conclusion
Overall, this national phase IV multicenter study comparing pembrolizumab with nivolumab showed no significant difference in efficacy in terms of either OS or PFS despite one of the drugs being used as first line therapy and the other as second line therapy. The study suggest that PD-1 inhibitors can have a relevant place as either first- or second-line treatment, and that patient performance affects outcome of the treatment.