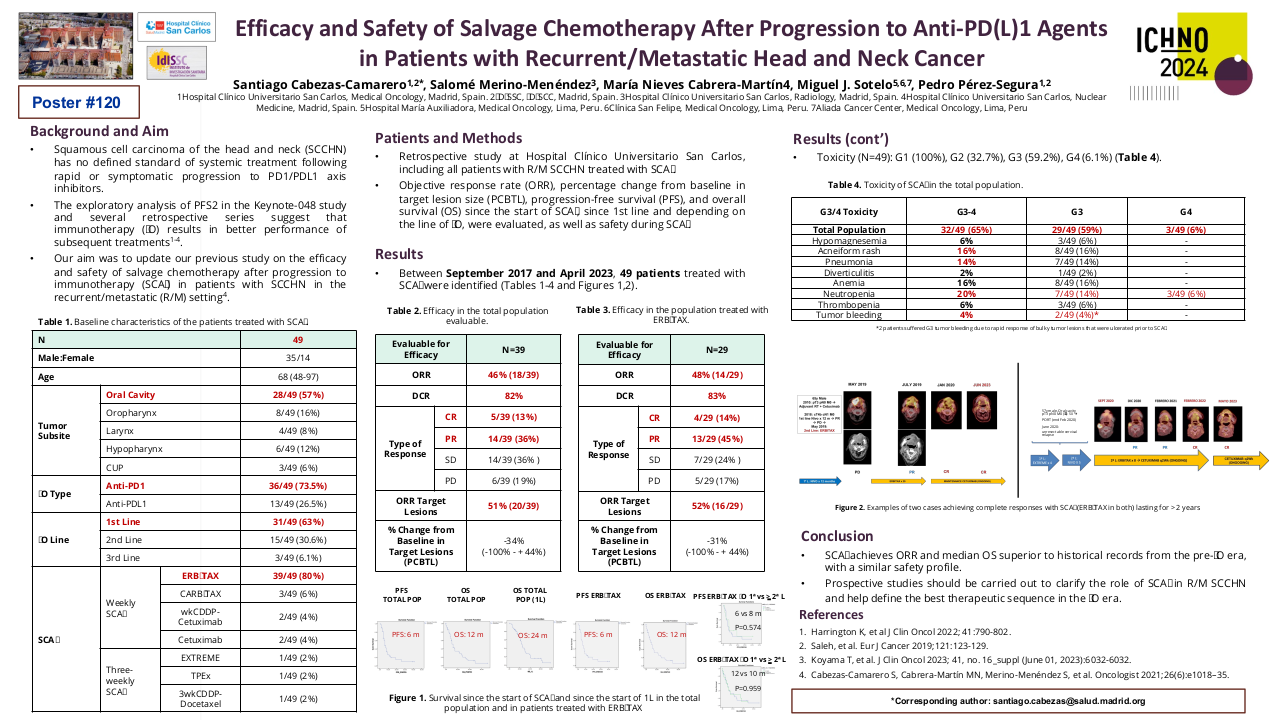
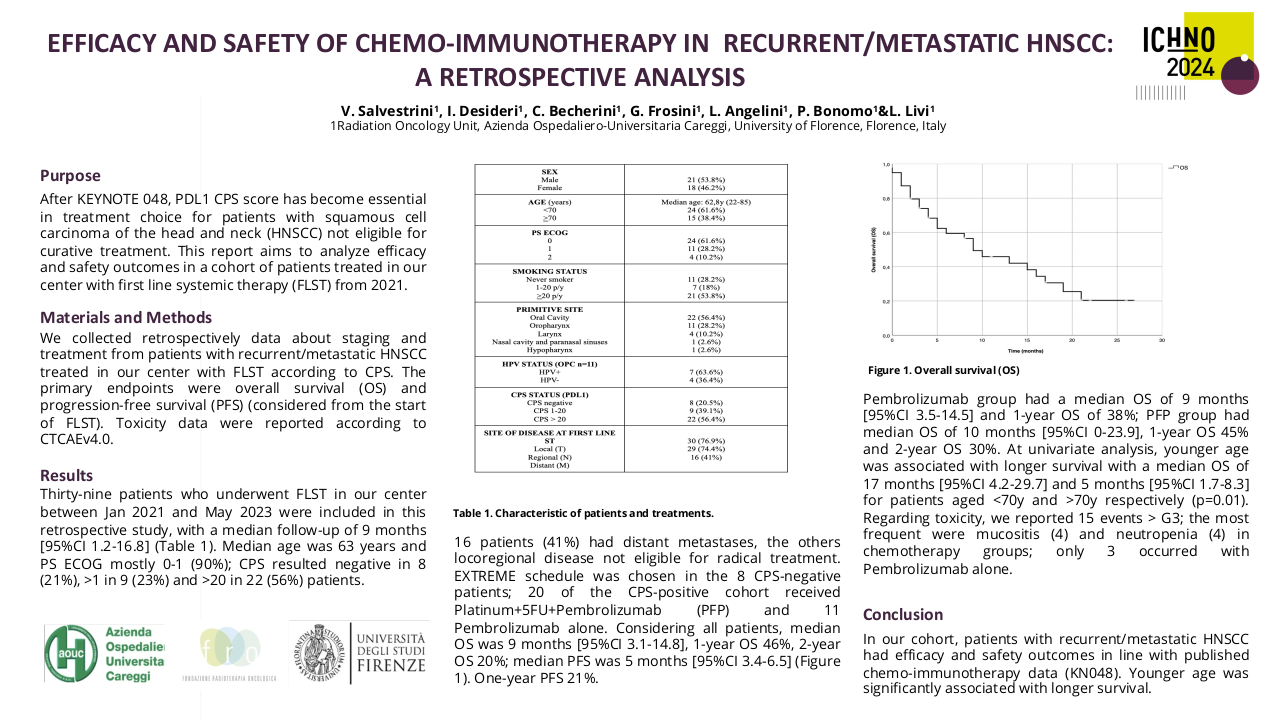
Efficacy and Safety of Salvage Chemotherapy After Progression to Anti-PD(L)1 Agents in Patients with Recurrent/Metastatic Head and Neck Cancer
Purpose/Objective
Squamous cell carcinoma of the head and neck (SCCHN) has no defined standard of systemic treatment following rapid or symptomatic progression to PD1/PDL1 axis inhibitors. The exploratory analysis of PFS2 in the Keynote-048 study and several retrospective series suggest that immunotherapy (IO) results in better performance of subsequent treatments. Our objective was to analyze the efficacy and safety of salvage chemotherapy after progression to immunotherapy (SCAI) in patients with SCCHN in the recurrent/metastatic (R/M) setting.
Material/Methods
Retrospective study at Hospital Clinico Universitario San Carlos, including all patients with SCCHN treated with SCAI. Objective response rate (ORR), percentage change from baseline in target lesion size (PCBTL), progression-free survival (PFS), and overall survival (OS) since the start of SCAI, since 1st line and depending on the line of IO, were evaluated, as well as safety during SCAI.
Results
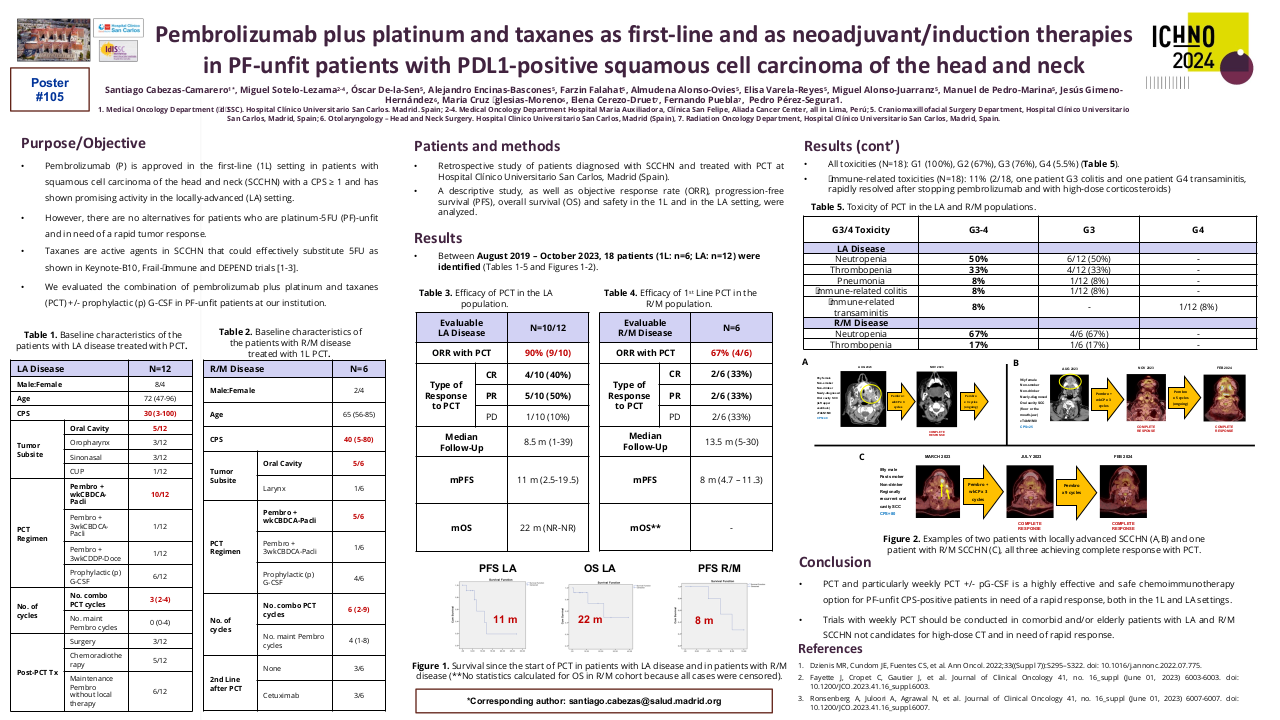
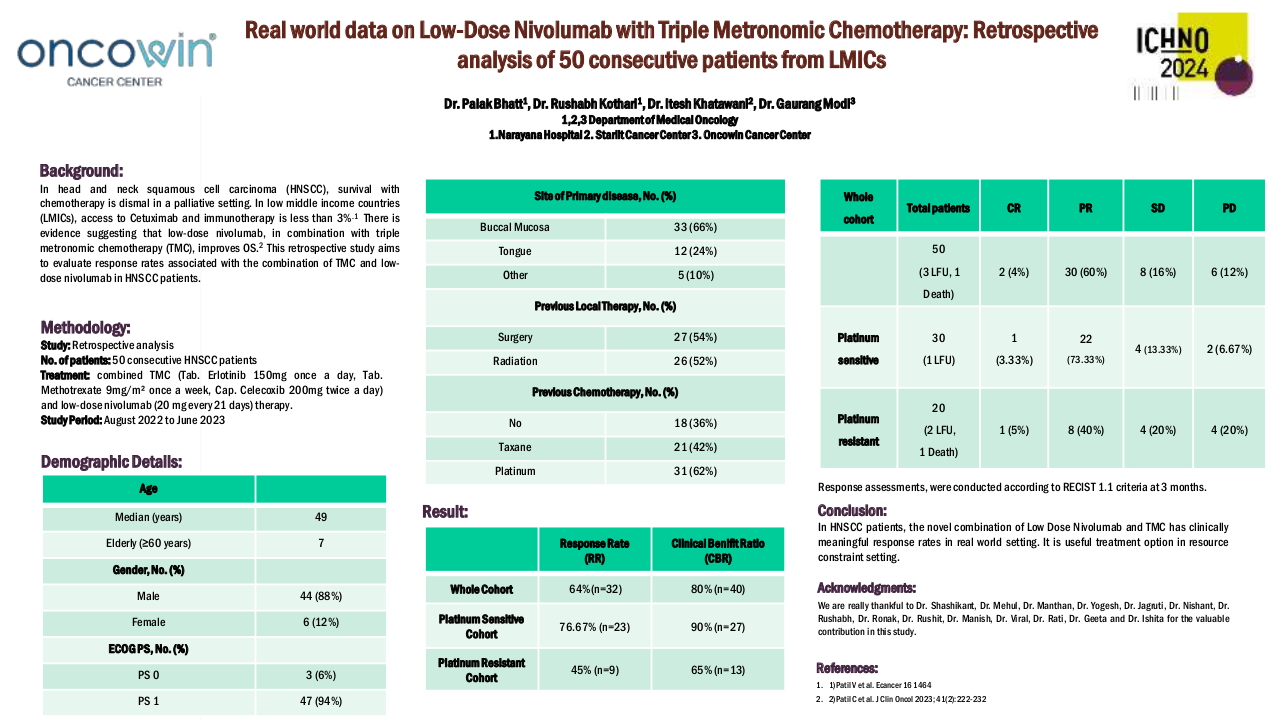
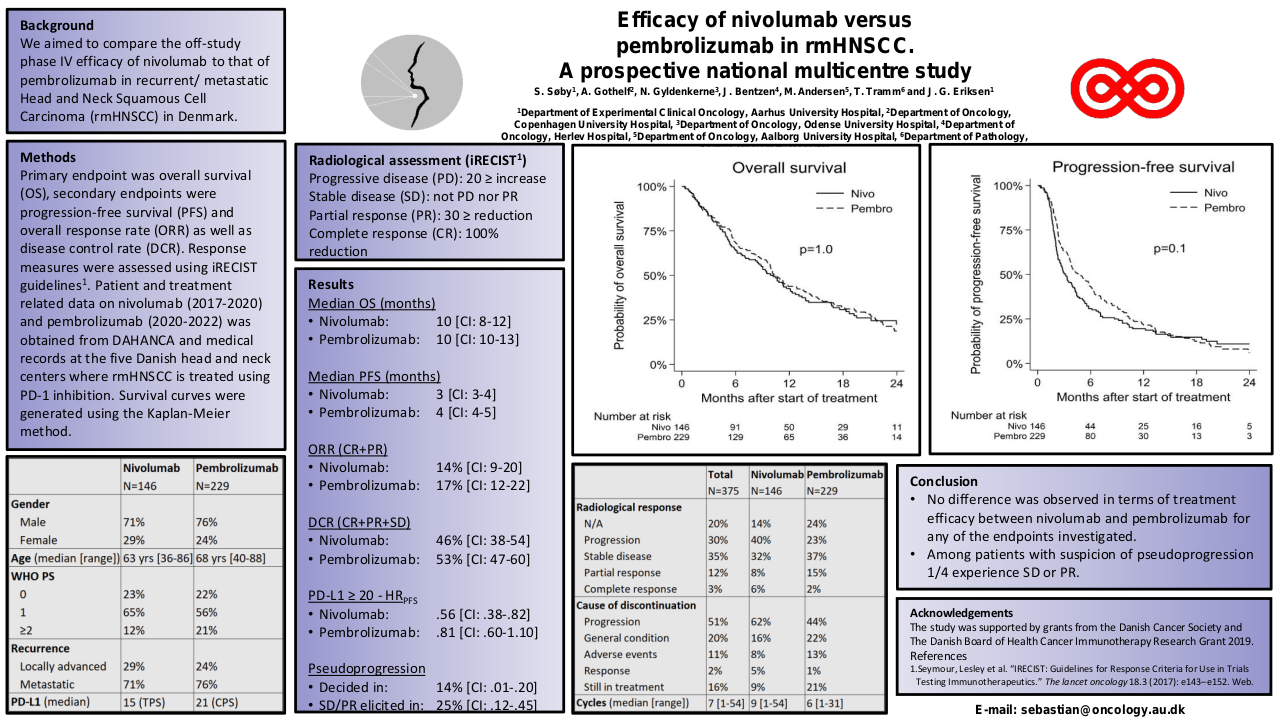
Between September 2017 and April 2023, 49 patients treated with SCAI were identified. M/F: 35/14. Age: 68 (48-97). Oral cavity/Oropharynx/Hypopharynx/Larynx/CUP: 8/28/4/6/3. Anti-PD1/Anti-PDL1: 36/13. IO 1st line/2nd line/3rd line: 31/15/3. SCAI (ERBITAX/CARBITAX/Cetuximab/wkCDDP-Cetuximab/EXTREME/TPEX/3wkCDDP-Docetaxel): 39/3/2/2/1/1/2. Total population efficacy (n=40): ORR=46% (CR=5, RP=14) and PCTLD= -34% (-100% to +44%). After 9 m of follow-up, the PFS and OS since the start of SCAI were 6 and 12 months, respectively, and after 21 m of follow-up since the start of 1st line, OS was 24 months. Efficacy of the population treated with ERBITAX (n=30): ORR=48%. After 9 m of follow-up, PFS=6 m and OS=12 m, with no differences in PFS (P=0.574) and OS (P=0.959) since SCAI, between patients treated with IO in 1st vs 2nd line. Toxicity (n=49): G1 (100%), G2 (32.7%), G3 (59.2%), G4 (6.1%).
Conclusion
SCAI achieves ORR and median OS superior to historical records from the pre-IO era, with a similar safety profile. Prospective studies should be carried out to clarify the role of SCAI in R/M SCCHN and help define the best therapeutic sequence in the IO era.
Harrington K, et al. Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study. J Clin Oncol 2022; 41:790-802.